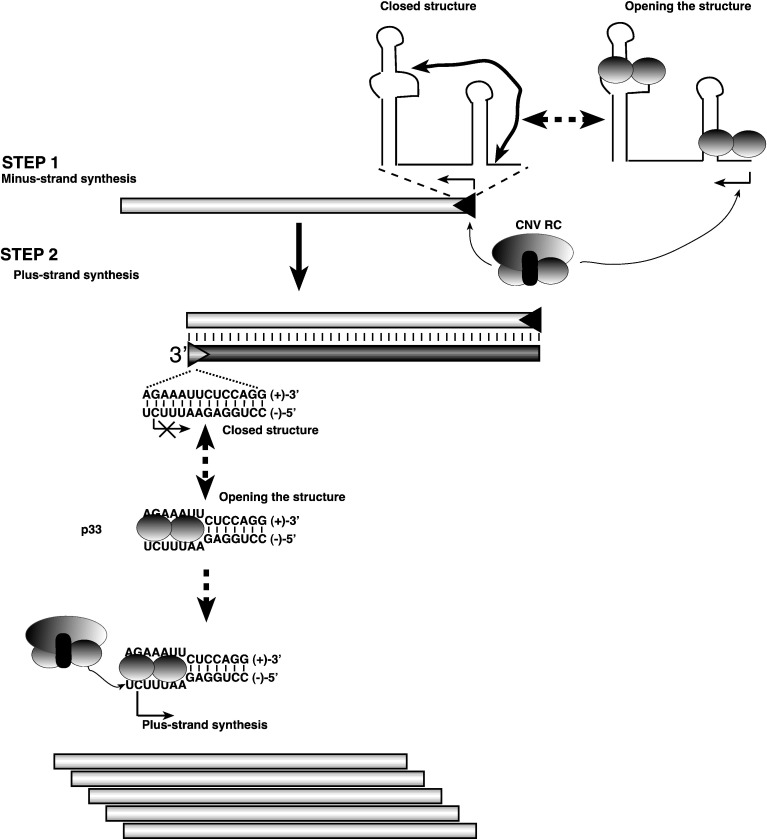
Fig. 8.
A model on the possible role of the RNA chaperone activity of p33 in tombusvirus replication. We predict that the RNA chaperone function of p33 replication protein is involved in opening the closed silencer–promoter structure, which then leads to minus-strand synthesis by the tombusvirus replicase (Step 1) However, the RNA chaperone activity of p33 is rather inefficient on the (+)RNA template, thus it is possible that host factors, such as elongation factor 1A, which is recruited for tombusvirus replication, might also be involved in minus-strand initiation. More importantly, p33 is likely involved in opening up the AU-rich terminus of the putative dsRNA replication intermediate (Step 2). We propose that binding of p33 to dsRNA could facilitate loading of the replicase to the open AU-rich end in the dsRNA replication intermediate, followed by initiation of plus-strand synthesis (bottom). It is likely that binding of p33 could stabilize the open structure within the AU-rich stretch in the cPR promoter as shown. After initiation, the tombusvirus replicase can efficiently unwind the remaining part of the dsRNA template during RNA synthesis as demonstrated earlier (Panavas et al., 2006).