Table 1.
Conditions optimization.
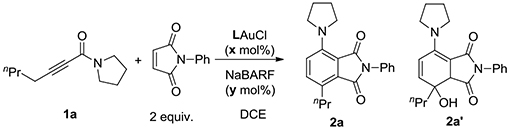
| ||||||
|---|---|---|---|---|---|---|
| Entry | L | x(%)/y(%) | temp/time | additive | conversion | 2a/2a’b |
| 1c | L1 | 5/10 | 80 °C/15 h | - | 7% | - |
| 2 | L1 | 5/10 | 80 °C/15 h | - | 76% | <2%/23% |
| 3 | L2 | 5/10 | 80 °C/15 h | - | 21% | - |
| 4 | L3 | 5/10 | 80 °C/15 h | - | 20% | <2%/8% |
| 5 | L4 | 5/10 | 80 °C/15 h | - | 54% | 18%/35% |
| 6 | PPh3 | 5/10 | 80 °C/15 h | - | 20% | - |
| 7 | Johnphos | 5/10 | 80 °C/15 h | - | 20% | - |
| 8 | WangPhos | 5/10 | 80 °C/15 h | - | 20% | - |
| 9 | L4 | 5/10 | 80 °C/15 h | 3Å MS | 25% | - |
| 10 | L4 | 5/10 | 80 °C/15 h | Boc2Od | 100% | 72%/26% |
| 11e | L4 | 5/10 | 80 °C/15 h | Boc2Od | 100% | 73%/26% |
| 12 | L4 | 5/10 | 80 °C/30 h | Boc2Od | 100% | 91%f/0% |
All the reactions were run in DCE (0.05 M) in sealed vials.
NMR yield using triisopropylbenzene as the internal reference.
No N-phenylmaleiimide was added.
2 equiv. used.
Reaction run under Ar.
86% isolated yield.
