FIG. 1-1.
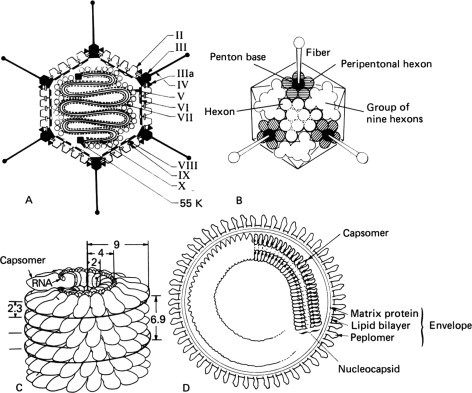
Features of virion structure, exemplified by adenovirus (A,B), tobacco mosaic virus (C), and paramyxovirus (D). Not to scale. (A,B) Icosahedral structure of adenovirion. All hexon capsomers are trimers of the same polypeptide (II), distinguished as “group of nine” or “peripentonal,” respectively, only by their location in the capsid. The penton base is a pentamer of polypeptide III; the fiber is a trimer of polypeptide IV. Several other viral polypeptides occur just beneath the capsid (VI, VIII, IX) and others again in the core (V, VII, 55K), where they are intimately associated with the viral DNA. (C) The structure of helical nucleocapsids has been elucidated by studies of a nonenveloped plant virus, tobacco mosaic virus, but the principles apply to animal viruses with helical nucleocapsids, all of which are enveloped. In tobacco mosaic virus a single polypeptide is folded to form a capsomer. A total of 2130 capsomers assemble in a helix with a pitch of 2.3 nm and an axial repeat of 6.9 nm (49 subunits in each three turns). The 6-kb RNA genome sits in a groove on the inner part of the capsomer, and is wound to form an RNA helix of the same pitch, 8 nm in diameter, which extends the length of the virion. The virion is 300 nm long and 18 nm in diameter, with a hollow cylindrical core 4 nm in diameter. (D) All animal viruses with a helical nucleocapsid and some of those with an icosahedral capsid are enveloped. The envelope consists of a virus-specified matrix protein (M; absent in Arenaviridae, Bunyaviridae, and Coronaviridae, as well as in the enveloped viruses with icosahedral capsids), beneath a lipid bilayer in which are inserted numerous glycoprotein peplomers. [A, from H. S. Ginsberg, In “Comprehensive Virology” (H. C. Fraenkel-Conrat and R. R. Wagner, eds.), Vol. 13,p. 409. Plenum Press, New York, 1979. B, by John Mack, from R. M. Burnett, In “Biological Macromolecules and Assemblies: Virus Structures” (F. Jurnak and A. McPherson, eds.), Vol. 1,p. 337. Wiley, New York, 1984; C, from C. F. T. Mattern, In “Molecular Biology of Animal Viruses” (D. P. Nayak, ed.), Vol. 1,p. 5. Dekker, New York, 1977; and D, modified from D. L. D. Caspar et al., Cold Spring Harbor Symp. Quant. Biol. 27,49 (1962).]
