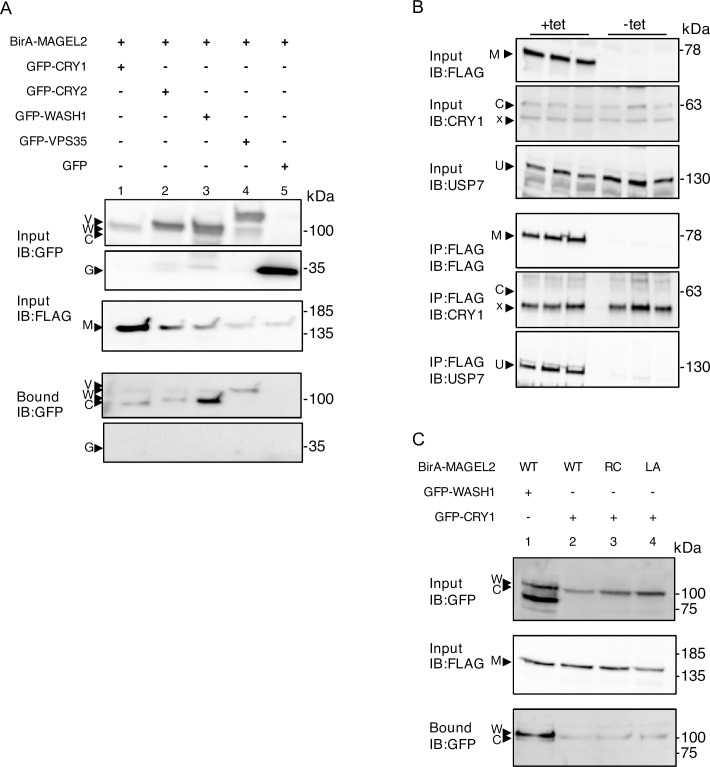
Fig 1. MAGEL2 is in proximity to CRY1 and CRY2 as detected by BioID.
A) U2OS cells were transiently transfected with cDNA constructs encoding epitope-tagged proteins and incubated with excess biotin. After 24 h, cell lysates were collected, an aliquot was retained as input, and the remaining sample was processed by streptavidin affinity purification to recover proteins biotinylated by BirA* fusion proteins (bound). Input and bound samples were immunoblotted (IB) to detect recombinant proteins. BirA*-MAGEL2 (M) was co-transfected with GFP-tagged CRY1 (C) or CRY2 (C), or with GFP-WASH1 (W) or GFP-VPS35 (V) as interacting positive controls. GFP alone was used as a non-interacting negative control (G). B) FLAG-MAGEL2 (M) expression was induced (+tet), or not induced (-tet) with tetracycline in stably transfected HEK cells, then a co-immunoprecipitation experiment was performed, in triplicate, to detect interactions between FLAG-MAGEL2 and endogenous USP7 (U) or endogenous CRY1 (C). A cross-reacting protein is detected with the anti-CRY1 antibody at a smaller molecular weight than the endogenous CRY1 protein (x). C) BirA*-MAGEL2 (wildtype, WT), BirA*-MAGEL2p.R1187C (RC), or BirA*-MAGEL2p.LL1031AA (LA) were co-transfected with GFP-CRY1 or GFP-WASH1 (positive control) and processed for BioID.