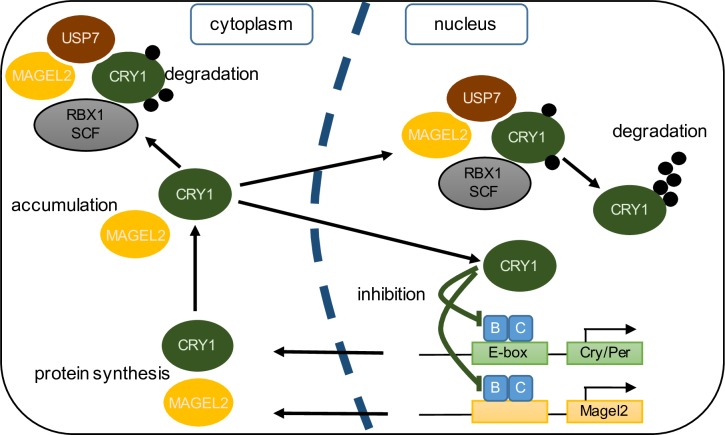
Fig 6. Simplified model for the role of MAGEL2 in circadian rhythm.
CLOCK and BMAL1 activate clock-controlled genes, including MAGEL2, CRY1/2 and PER1/2/3 by binding to elements, such as E-box motifs, in their promoters. Along with PER proteins (not shown), CRY proteins take part in a feedback loop by inhibiting their own expression through repression of CLOCK/BMAL1 activity. CRY1 is modified post-translationally by ubiquitination (black circles). A complex consisting of a RING finger protein (RBX1) and a SKP-Cullin-F-Box complex facilitates ubiquitination of CRY1, while deubiquitinases, including USP7, deubiquitinate CRY1. The balance between ubiquitination and deubiquitination, and between stability and proteasomal degradation, is regulated by various regulatory proteins including the MAGE protein MAGEL2. Translocation between the cytoplasm and the nucleus, and other post-translational modifications such as phosphorylation also modulate the rate of degradation of CRY1 protein.