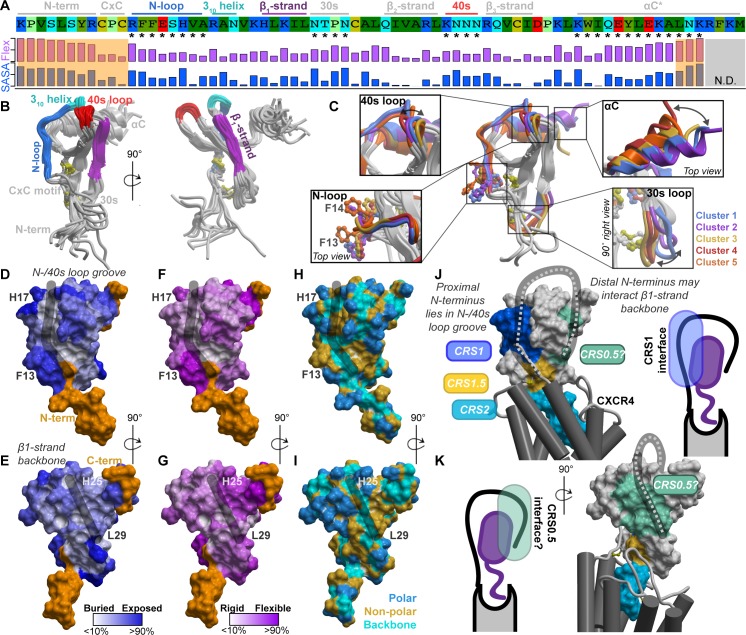
Fig 1. The structural and physicochemical properties of the chemokine CXCL12.
(A) The sequence of CXCL12, its annotated structural domains, and quantification of side chain conformational variability and normalized SASA. The values are given in S2 Table. (B) The exposed structural elements of CXCL12 highlighted on the crystallographic ensemble of structures. (C) Coordinated conformations of CXCL12 structural elements, which are indicated by asterisks in panel A, captured by clustering of the 30s loop. (D–G) Normalized residue side chain SASA (D–E) and side chain conformational variability (F–G) mapped onto the CXCL12 surface. The highly flexible N- and C-termini are colored orange. (H–I) Exposed residue backbones, polar and nonpolar side chains on the CXCL12 surface. In panels D–I, the black strokes highlight surface grooves in CXCL12. (J–K) A coarse-grain hypothesis of CXCR4 (gray) bound to CXCL12 (surface mesh is colored by known and proposed CRS between the receptor and chemokine). The hypothesis is based on published CRS2 models and the presented structural and physicochemical properties of CXCL12. CRS, chemokine recognition site; SASA, solvent-accessible surface area.