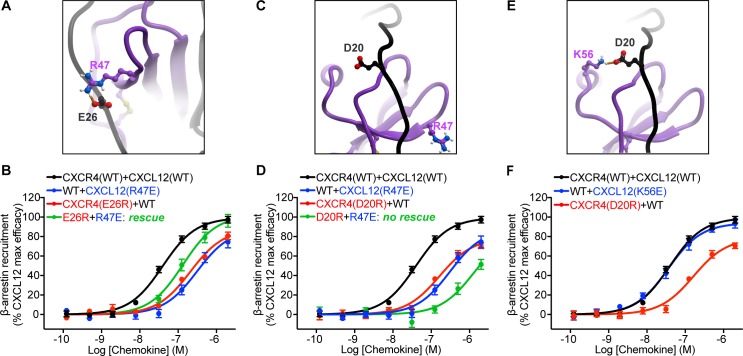
Fig 5. Validation of predicted interactions in CRS1 via loss-of-function and reciprocal charge-reversal substitutions in a BRET2-based β-arrestin recruitment assay.
(A) The predicted interaction between CXCR4 E26 and CXCL12 R47. (B) Individual charge reversals (E26R in CXCR4 or R47E in CXCL12) lead to substantial deficits in both potency and efficacy of β-arrestin recruitment; however, when these mutants are combined, the deficits are partially rescued. (C) According to the model, CXCR4 D20 and CXCL12 R47 are located 15.8 Å away from each other and do not interact. (D) Combining CXCR4(D20R) and CXCL12(R47E) does not rescue signaling deficits caused by each of these mutations individually. (E) The predicted interaction between CXCR4 D20 and CXCL12 K56. (F) Unlike CXCR4(D20R), the K56E mutation in CXCL12 does not cause any detectable signaling defects, therefore, functional rescue was not attempted. Data represent at least n = 3 independent biological replicates. The mean and SEM is reported for each point. WT curves were obtained in parallel with each respective mutant. The underlying numerical data for each figure panel can be found in S1 Data. BRET, bioluminescence resonance energy transfer; CRS, chemokine recognition site; WT, wild type.