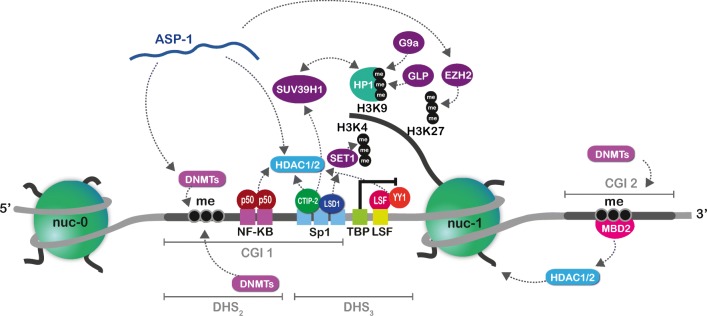
Fig. 3.
Epigenetic control of HIV-1 silencing during latency. HIV-1 silencing during latency is regulated through epigenetic mechanisms. During latency, transcription factors redundantly recruit histone modifiers. For instance, the negatively acting NF-κB homodimer p50-p50 occupies cognate binding sites in the 5′ LTR and recruits HDAC1 and HDAC2. In microglial cells, the cellular factor CTIP2 represses HIV-1 gene expression at least through three distinct modes. One of them is depicted in the figure. CTIP2 and LSD1 bind the Sp1 sites in the 5′ LTR. CTIP2 sequentially recruits HDACs and the HMT SUV39H1 that catalyses H3K9me3. This mark is recognized by HP1 that recruits further SUV39H1 units spreading the heterochromatic H3K9me3 mark. In parallel, LSD1 recruits the hCOMPASS complex, notably containing the histone methyltransferase (HMT) SET1 that stimulates H3K4me3. Furthermore, several HMTs (including G9a, GLP and EZH2) are responsible for depositing H3K9me2/3 and H3K27me3, respectively; their mode of recruitment to the HIV-1 promoter remains unclear. Two CpG islands (CGIs) surround the HIV-1 TSS and are heavily methylated by DNA methyltransferases (DNMTs), allowing the recruitment of MBD2 and the associated NuRD complex (containing HDAC2) to the second CGI. Recent reports show that the HIV-1-encoded antisense transcript ASP-1 also contributes to epigenetic silencing through promoting recruitment of Dnmt3, HDAC1 and EZH2 to the 5′ LTR