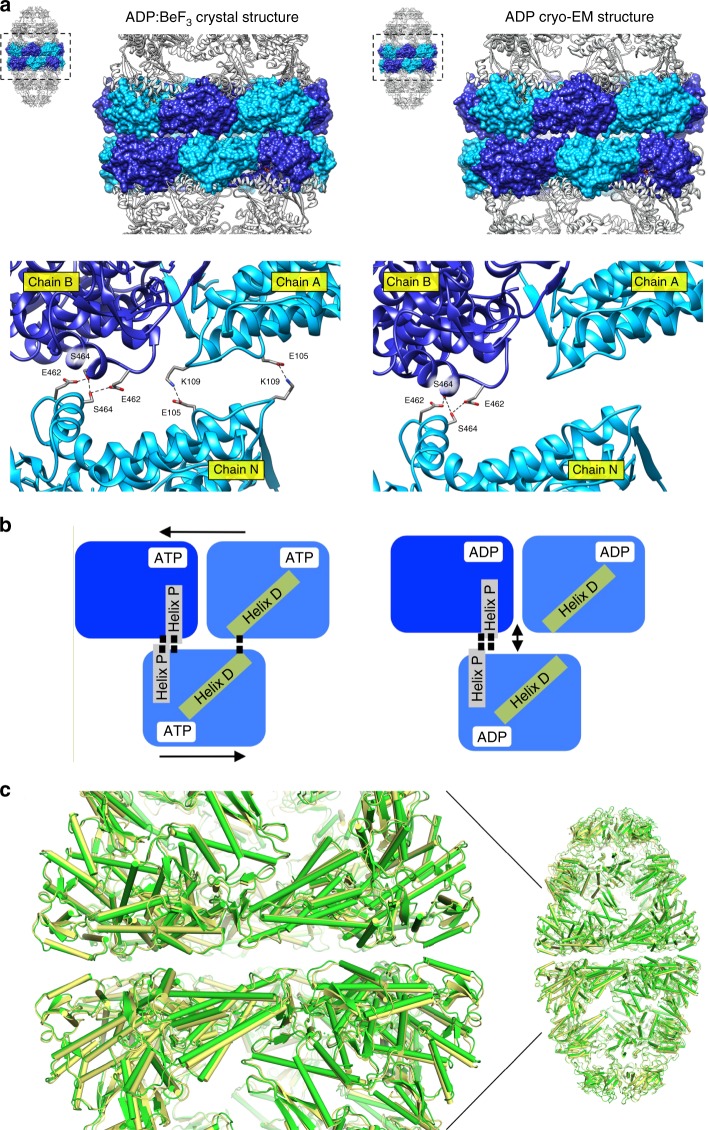
Fig. 4. mHsp60 inter-ring interface of mHsp60–mHsp10 football complexes.
a Side views of the ADP:BeF3 (left) and the ADP (right) footballs. The equatorial domains of the mHsp60 subunits are rendered as a molecular surface in alternating blue and light blue. The other domains in the mHsp60 subunits and mHsp10 subunits are presented as ribbon diagrams. Below is a close-up view of the staggered arrangement of two mHsp60 subunits in one ring contacting one subunit in the opposite ring represented as ribbon diagrams. Residues engaged in inter-ring contacts are presented as sticks. In the ADP:BeF3 football, these are E105, K109, E462, and S464 (left). In the ADP football, these are S464 and E462 (right). Black dashed lines denote bonds between the interacting residues. b Cartoon representing the differences in the interactions between left (Helix D) and right (Helix P) contact sites of the mHsp60 subunits across each ring of the ADP:BeF3 (left) and ADP (right) footballs. c Superposition of the ADP:BeF3 (green) and ADP (yellow) football structures showing a close-up of the mHsp60 inter-ring region.