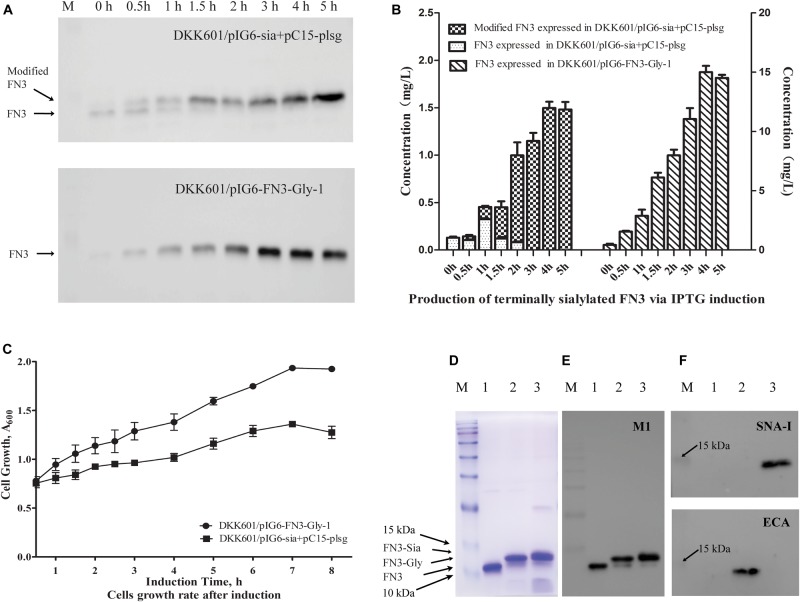
FIGURE 2.
Production of terminally sialylated homogeneous N-glycosylated FN3 in the E. coli periplasm. (A) Western-blot analysis of the expression of modified FN3. E. coli cell lysates containing pIG6-Sia + pC15-plsg (upper panel) or pIG6-FN3-Gly-1 plasmids (lower panel) were detected at the indicated induction time points using anti-FLAG M1 antibody, with quantification of protein yields at different induction time points to determine optimal expression conditions (For detailed information, see Supplementary Figures 3, 4) (B). (C) Growth curves of E. coli DKK601/pIG6-FN3-Gly-1 (expressing FN3 protein without sialylation pathway) and E. coli DKK601/pIG6-Sia + pC15-plsg (expressing modified FN3 protein with sialylation pathway) cells after induction of recombinant protein expression and glycosylation. Purified unmodified FN3, N-glycosylated FN3 (FN3-Gly) and sialylated FN3 (FN3-Sia) produced over 5 h at 28°C in E. coli DKK601 cells containing the FN3 gene alone; FN3 and lsgCDEF, wecA, pglB and pglK genes for N-glycosylation (FN3-Gly) (Ding et al., 2017); or additional neuBCA and pl-ST6 genes to effect sialylation (FN3-Sia), detected by (D) Coomassie-stained SDS-PAGE, (E) Western blotting using anti-FLAG M1 antibody, and (F) lectin blotting using Neu5Ac-α-2,6-Gal/GalNAc-specific SNA-I (upper panel) or Gal-β-1,4-GlcNAc-specific ECA (lower panel) lectins. (D–F) lane 1–3: purified FN3, FN3-Gly, FN3-Sia. (For detailed information, see Supplementary Figure 5). Data are the means ± standard errors (SD) from three independent representative experiments.