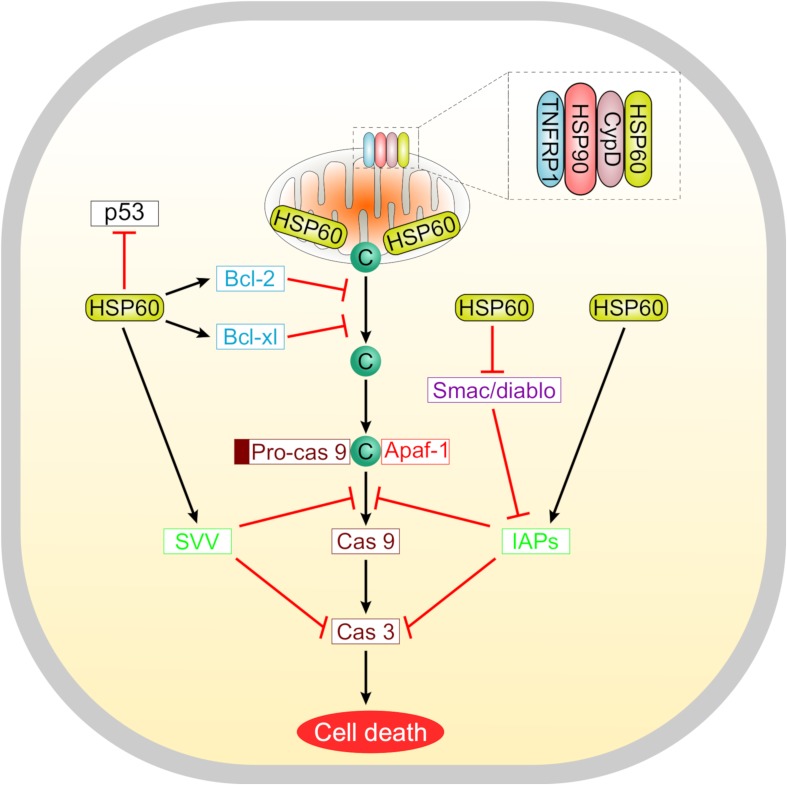
FIGURE 2.
A schematic representation summarizing the roles of HSP60 in regulating tumor cell apoptosis. Suppression of apoptosis by HSP60 is associated with increased levels of anti-apoptotic proteins like Bcl-2, Bcl-xL, and survivin. These molecules counteract the release of cytochrome c from mitochondria. Selectively in tumor cells, HSP60 forms a multichaperone complex with Cyclophilin D (Cyp D) and other chaperones including HSP90 and tumor necrosis factor receptor-associated protein 1 (TNFRP1) to maintain the mitochondrial permeability transition. Oncogenic HSP60 interacts with p53 in tumor cells and suppresses its action. HSP60 also controls the progression of apoptosis via regulating the mitochondrial release of smac/diablo and controlling the function of inhibitor of apoptosis (IAP) family proteins that inhibit caspases.