Figure 1.
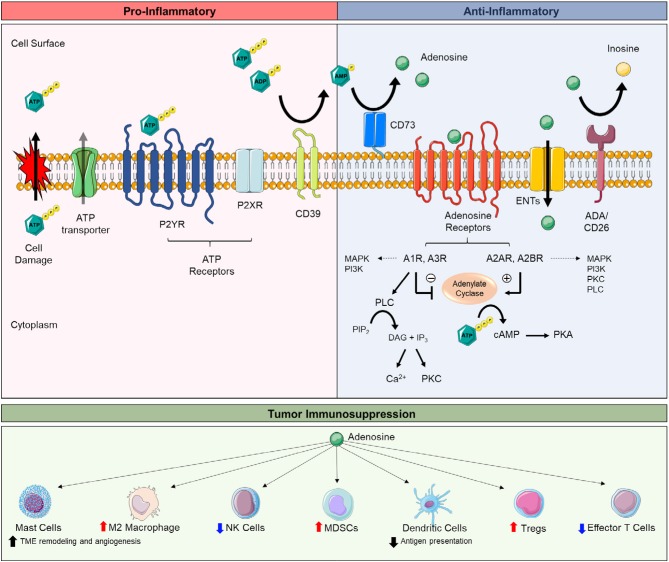
Extracellular adenosine synthesis, adenosine receptor signaling, and adenosine-mediated immunosuppression. Extracellular adenosine and receptor signaling is part of a large cascade of ecto-enzymes (e.g., CD39, CD73), membrane transporters (e.g., ENTs), and G-protein-coupled (e.g., P2YR, adenosine receptors) and ionotrophic receptors (e.g., P2XR) known as the purinergic pathway. The purinergic pathway mediates both pro-inflammatory and anti-inflammatory responses. The breakdown of extracellular adenosine triphosphate (ATP) to extracellular adenosine is key to balancing tissue inflammation. Intracellular ATP is released by lytic (e.g., stressed and/or apoptotic/necrotic cells) and non-lytic (e.g., pannexin-1 and connexins) routes secondary to tissue damage, inflammation, and/or hypoxia. Once released, ATP activates ATP receptors (e.g., P2XR and P2YR) to promote pro-inflammatory responses, including the release of inflammatory cytokines promote lymphocyte proliferation, cell mobility, and phagocyte recruitment. ATP is dephosphorylated to extracellular adenosine by CD39, converting ATP and adenosine diphosphate (ADP) to adenosine monophosphate (AMP), and CD73, converting AMP to adenosine. Extracellular adenosine signaling through adenosine receptors (e.g., A1R, A2AR, A2BR, A3R) promotes anti-inflammatory responses, including the release of pro-tolerance cytokines, regulatory lymphocytes, and skewing toward M2 macrophages. Extracellular adenosine also can be taken up intracellularly by equilibrative nucleoside transporters (e.g., ENTs) or be further metabolized to inosine (e.g., ADA/CD26). A2AR and A2BR signaling stimulate adenylate cyclase to produce cyclic AMP (cAMP) which activates protein kinase A (PKA). A1R and A3R signaling inhibit adenylate cyclase. Adenosine receptors can activate multiple signaling pathways (e.g., MAPK, PI3K, PLC, PKC, ion channels), depending on cell and tissue types. Tumors exploit the anti-inflammatory actions of extracellular adenosine to evade antitumor immune cells. A3R activation on mast cells promotes tumor microenvironment (TME) remodeling and angiogenesis, increases the population of M2 macrophages, and promotes the accumulation of myeloid-derived suppressor cells (MDSCs) in tumors. A2AR activation on T regulatory cells (Tregs) enhances their immunosuppressive activity (e.g., suppressing effector T cells). A2AR and/or A2BR activation on natural killer (NK) cells, dendritic cells, and effector T cells dampens the antitumor activity of these cells. Abbreviations: ectonucleoside triphosphate diphosphohydrolase-1 (CD39), ecto-5′nucleotidase (CD73), adenosine deaminase (ADA), phospholipase C (PLC), protein kinase C (PKC), diacylglycerol (DAG), phosphatidylinositol 4,5-bisphosphate (PIP2), inositol trisphosphate (IP3), mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K).