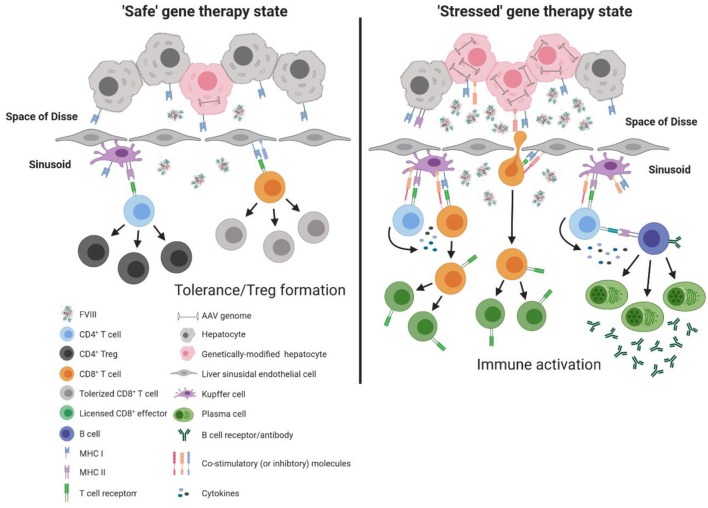
Figure 2.
Model of immune response to liver directed AAV-fVIII gene therapy. The liver is a unique immunoprivileged site that, through complex interactions of an array of liver immune constituents, teeters between tolerance and inflammation. These immune populations include liver sinusoidal endothelial cells (LSECs) that line the wall of the sinusoids and are intimately associated with resident macrophages of the liver (Kupffer cells), hepatic stellate cells (Ito cells) that reside in the space of Disse between hepatocytes and LSECs, and hepatic dendritic cells that reside in the sinusoidal lumen of the liver. Under basal conditions, an array of immune constituents (e.g., Kupffer cells and LSECs) express low levels of MHC and co-stimulatory molecules as well as immunomodulatory cytokines. In the absence of cellular stress following AAV-fVIII gene therapy (“safe” gene therapy state), the local immunomodulatory milieu of the liver can suppress the activation of vector specific and fVIII reactive T cells. Moreover, expression of co-inhibitory molecules by LSECs can aid in the efficient differentiation of fVIII specific Tregs. However, a bolus infusion of AAV particles and/or overexpression of fVIII can lead to cellular stress that possesses the capacity to deviate the immune environment from immunomodulatory to pro-inflammatory. Under AAV-fVIII gene therapy mediated cellular stress (“stressed” gene therapy state), genetically modified hepatocytes can up-regulate MHC class I and co-stimulatory molecules as well as the production of pro-inflammatory cytokines. CD8+ T cell recognition of cognate antigens expressed by “stressed” hepatocytes can be activated, ultimately resulting in the cytolysis of genetically modified hepatocytes and decline in fVIII production. In addition, the pro-inflammatory milieu generated from cellular stress can promote differentiation of effector fVIII specific CD4+ T cells that can help activate fVIII specific B cells for formation of inhibitors.