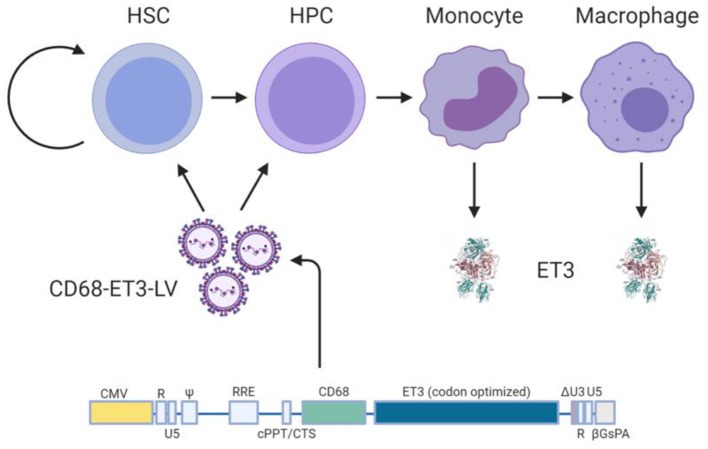
Figure 4.
Ex vivo CD68-ET3-LV CD34+ clinical gene therapy paradigm. Autologous CD34+ HSPC are isolated from subjects with hemophilia A, genetically modified ex vivo using LV encompassing a codon optimized pfVIII transgene (ET3) under the monocyte lineage restricted promoter, CD68. Genetically modified HSPCs are then infused back into the subject following non-myeloablative conditioning with immune suppression. Post-administration of the genetically-modified autologous cell product, plasma fVIII levels, vector copy number in peripheral blood, and fVIII immunity status are followed.