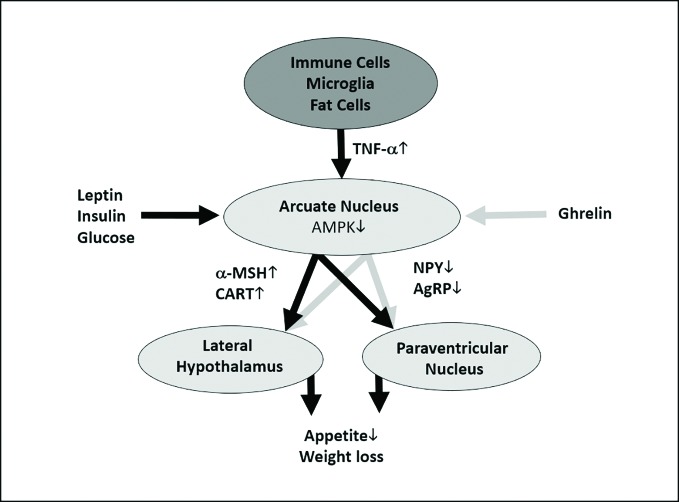
Figure 5.
Hypothetical and simplified model of how tumor necrosis factor alpha (TNF-α) could cause anorexigenic effects. TNF-α is released by immune cells, microglia, fat cells, and many other cells (Perskidskiĭ and Barshteĭn, 1992). TNF-α unfolds its anorexigenic effects at the arcuate nucleus of the hypothalamus, which is a central regulator of energy homeostasis, by inducing the production α-melanocyte-stimulating hormone (α-MSH) and cocaine- and amphetamine-regulated transcript (CART) in proopiomelanocortin (POMC)-expressing neurons; additionally, it leads to a decreased production of the orexigenic signals agouti-related protein (AgRP) and neuropeptide Y (NPY) in AgRP-expressing neurons (Romanatto et al., 2007). As TNF-α has been shown to stimulate the intracellular AMP-activated protein kinase (AMPK) (Tse et al., 2017), which integrates orexigenic and anorexigenic signals within the arcuate nucleus (Minokoshi et al., 2004), we hypothesize that this mechanism might play a role in the upregulation of α-MSH and CART and the downregulation of AgRP and NPY. These molecular signals will be conveyed to the lateral hypothalamus and the paraventricular nucleus and thus lead to reduced appetite and weight loss (Claret et al., 2007). However, orexigenic (e.g., ghrelin) and anorexigenic (e.g., glucose, insulin, and leptin) signals from the body periphery modify AMPK activity at the arcuate nucleus (Minokoshi et al., 2004). As mentioned above, this is a simplified figure which neglects important mechanisms influencing the release and the effects of TNF-α. For example, ghrelin can alter TNF-α signaling at cellular level (Himmerich and Sheldrick, 2010). Anorexic signals are depicted as black, orexigenic signals as gray arrows. The dark gray oval represents the entirety of TNF-α-producing cells, the light gray ovals show hypothalamic areas important for appetite and weight regulation.