Abstract
Objectives
Specific systemic autoimmune syndrome characterized by inflammatory myopathy, arthritis or arthralgias, interstitial lung disease (ILD), fever, Raynaud’s phenomenon, and mechanic’s hands is called antisynthetase syndrome (AS). The aim of this study was to assess the clinical spectrum associated with presence of aminoacyl-transfer RNA synthetase autoantibodies (ASA).
Material and methods
A total of 305 patients with presence of myositis-specific autoantibodies were identified in the database of immunological tests performed in the Clinical Immunology and Transplantology Unit, Medical University of Gdansk between January 2011 and March 2016. In 110 patients (36%) ASA were detected. The detailed analysis included 50 patients with ASA for whom full clinical data were available.
Results
The incidence of specific ASA in the analyzed group was: Jo-1 46% (23 patients), PL-12 32% (16 patients), PL-7 16% (8 patients), OJ 12% (6 patients), EJ 6% (3 patients). In 10% (5 patients) there was coexistence of at least one ASA, and in another 5 patients there was coexistence of ASA with other antibodies specific for myositis (MSA). In the analyzed group of patients 11 (22%) satisfied the Bohan and Peter criteria for dermatomyositis, 1 for polymyositis. In 5 patients (10%) based on clinical presentation and ASA presence the AS was recognized. Another 3 patients met the criteria of the overlap syndrome polymyositis respectively with systemic lupus, rheumatoid arthritis, and scleroderma. In 5 patients undifferentiated connective tissue disease was diagnosed, and 14 consecutive patients were diagnosed with other connective tissue diseases, while 12 patients did not receive a definitive diagnosis.
Conclusions
The clinical presentation of patients with the presence of ASA is varied. Their presence indicates not only idiopathic inflammatory myopathies, but also non-specifically other disease entities. These patients require observation for the development of idiopathic inflammatory myopathy, and ILD.
Keywords: antisynthetase syndrome, antisynthetase antibodies, idiopathic inflammatory myopathy
Introduction
Idiopathic inflammatory myopathies (IIMs) are a heterogeneous group of acquired autoimmune diseases [1–4]. Based on their clinical and histopathological features, IIMs can be classified as polymyositis (PM), dermatomyositis (DM), inclusion body myositis and immune-mediated necrotizing myopathy [5]. Myositis may also be associated with malignancy (cancer-associated myositis – CAM) and form overlap syndromes with other connective tissue diseases.
Idiopathic inflammatory myopathies are diagnosed by progressive proximal muscle weakness, characteristic skin lesions, raised serum creatine kinase, characteristic electromyographic abnormalities, evidence of muscle inflammation or necrosis. Other organs are frequently affected in IIMs, such as the skin, joints, lungs, gastrointestinal tract and heart.
Autoantibodies are detectable in the sera of 50–80% of PM/DM patients and consist of myositis-associated and myositis-specific antibodies (MAAs and MSAs, respectively) [6]. The MAAs are not specific to PM/DM and are found in a variety of autoimmune diseases. The exact role of these autoantibodies in the disease pathology is still not clear.
Specific systemic autoimmune syndrome characterized by inflammatory myopathy, arthritis or arthralgias, interstitial lung disease (ILD), fever, Raynaud’s phenomenon, and mechanic’s hands is called antisynthetase syndrome (AS). It is frequently considered to be an entity overlapping with DM or PM [5].
Antisynthetase syndrome is strongly associated with the presence of autoantibodies to aminoacyl-transfer RNA (tRNA) synthetases (antisynthetase antibodies – ASA). They belong to the family of cytoplasmic enzymes that catalyze the binding of specific amino acids to the matching tRNA during the translation phase of protein synthesis [7] and are considered to be myositis-specific antibodies.
There are currently eight identified autoantibodies that target the amino-acyl tRNA synthetase enzymes: anti-Jo-1 (anti-histidyl tRNA synthetase), PL-12 (anti-alanyl tRNA synthetase), PL-7 (anti-threonyl tRNA synthetase), EJ (anti-glycyl tRNA synthetase), OJ (anti-isoleucyl tRNA synthetase), KS (asparaginyl-tRNA synthetase), and the more recently identified Ha (tyrosyl-tRNA synthetase) and Zo (phenylalanyl-tRNA synthetase). The presence of ASA in combination with the typical clinical presentation gives rise to the diagnosis of AS.
The aim of this study was to assess the clinical spectrum associated with presence of ASA. Particularly, we were interested how many patients have clinical symptoms of the AS.
Material and methods
A total of 305 patients with presence of MSAs were identified in the database of immunological tests performed in the Clinical Immunology and Transplantology Unit, Medical University of Gdansk between January 2011 and March 2016. In 110 patients (36%) ASA were detected. Because the laboratory is a reference center for many hospitals, the detailed analysis included 50 patients with ASA for whom full clinical data were available.
This retrospective work concerned data from a clinical database; no consent from the bioethics committee was required.
Results
The incidence of specific ASA in the analyzed group was: Jo-1 46% (23 patients), PL-12 32% (16 patients), PL-7 16% (8 patients), OJ 12% (6 patients), EJ 6% (3 patients). Antibodies KS (asparaginyl), Ha (tyrosyl) and Zo (phenylalanyl) were not detected.
In 12% (6 patients) the coexistence of at least one ASA, and in another 5 patients the coexistence of ASA with other MSA was found: anti-Mi-2 (antibodies to nuclear helicase) – 2 patients, SRP (antibodies to signal recognition particle) – 1 patient, TIF1g (antibodies to 155-kD nuclear protein transcriptional intermediary factor [TIF]-1gamma) – 1 patient, MDA5 (antibodies to melanoma differentiation-associated gene 5) – 1 patient.
The 68% of patients also had MAAs. The most detected MAA was anti-Ro-52 (48%, 24 patients), followed by Ku (22%, 11 patients), PM75 (12%, 6 patients), PM100 (6%, 3 patients).
In the analyzed group of patients with the presence of ASA, 11 (22%) satisfied the Bohan and Peter criteria for DM, 1 for PM [8]. In five patients (10%) based on clinical presentation and ASA presence the AS was recognized. Another three patients met the criteria of the overlap syndrome PM respectively with systemic lupus, rheumatoid arthritis, and scleroderma.
In 5 patients undifferentiated connective tissue disease was diagnosed, and 14 consecutive patients were diagnosed with other connective tissue diseases (scleroderma in 2 and vasculitis in another 2 patients; individual patients were diagnosed with lupus, rheumatoid arthritis, psoriatic arthritis, chronic eosinophilic pneumonia, cutaneous mastocytosis, Sjögren’s syndrome, multiple sclerosis, sarcoidosis, idiopathic ILD). Eleven patients did not receive a definitive diagnosis (Table I).
Table I.
Incidence of antisynthetase antibodies in individual disease entities in analyzed group of 110 patients
| Disease | Antibody | ||||
|---|---|---|---|---|---|
| Jo-1 | PL-7 | PL-12 | OJ | EJ | |
| Idiopathic inflammatory myopathies | |||||
| Dermatomyositis | 7 | 1 | 1 | 3 | – |
| Polymyositis (PM) | 1 | – | – | – | – |
| Antisynthetase syndrome | 4 | – | 1 | 1 | – |
| Overlap syndrome | |||||
| PM/SLE | – | + | + | + | – |
| PM/RA | – | – | + | – | – |
| PM/scleroderma | – | – | – | – | + |
| Other clinical diagnosis | |||||
| Undifferentiated connective tissue disease | – | 2 | 2 | – | 1 |
| Other diseases* | 5 | 2 | 6 | 1 | 2 |
| No final diagnosis | 6 | 2 | 3 | – | – |
Jo-1 – anti-histidyl tRNA synthetase, PL-7 – anti-threonyl tRNA synthetase, PL-12 – anti-alanyl tRNA synthetase, OJ – anti-isoleucyl tRNA synthetase, EJ – anti-glycyl tRNA synthetase, SLE – systemic lupus erythematosus, RA – rheumatoid arthritis
scleroderma, vasculitis, lupus, rheumatoid arthritis, psoriatic arthritis, chronic eosinophilic pneumonia, cutaneous mastocytosis, Sjögren’s syndrome, multiple sclerosis, sarcoidosis, idiopathic interstitial lung disease.
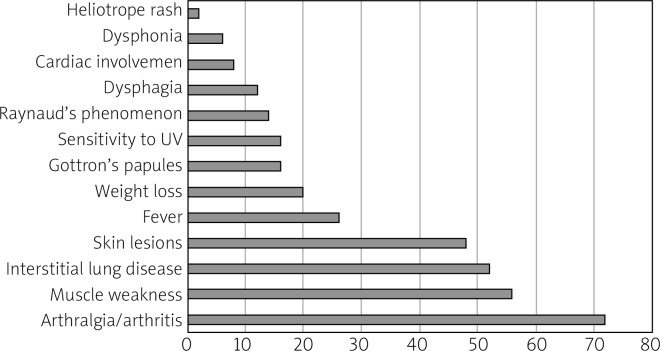
The average age of onset in patients diagnosed with IIM was 48 years (range 18–84 years); 76% were women. 56% of patients with the presence of ASA had muscle weakness, 72% arthralgia (36.1% had active arthritis), 48% skin lesion, 26% fever, 20% weight loss, 16% Gottron’s papules, 14% Raynaud’s phenomenon, 14% sensitivity to UV, 12% dysphagia, 8% cardiac involvement, 6% dysphonia and 2% heliotrope rash.
52% (26 patients) had ILD. The incidence of ASA in this group of patients was: Jo-1 42.3%, PL-12 30.7%, OJ 23%, PL-7 19.2%. Laboratory markers of muscle damage (elevated creatine kinase) were found mainly in patients with the presence of anti-Jo-1 (55% of patients) (Fig. 1 and Table II).
Fig. 1.
Clinical manifestations of patients with the presence of antisynthetase autoantibodies as a percent of patients in analyzed group.
Table II.
Clinical manifestations depending on the incidence of the type of anti-ARS antibodies (ASA) in the group of 50 analyzed patients
| Type of antibody | n | Muscle weakness n (%) | Body mass loss n (%) | Joint involvement n (%) | Pulmonary involvement n (%) | Skin involvement n (%) | Heart involvement n (%) | Dysphagia n (%) | CK elevation n (%) | CRP elevation n (%) | Fever n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jo-1 | 23 | 11 (47.8) | 2 (8.7) | 15 (65.2) | 11 (47.8) | 14 (60.8) | 2 (8.7) | 1 (4.3) | 10 (43.4) | 10 (43.4) | 6 (26) |
| PL-7 | 8 | 5 (62.5) | 3 (37.5) | 4 (50) | 5 (62.5) | 3 (37.5) | 1 (12.5) | 2 (25) | 3 (37.5) | 2 (25) | 3 (37.5) |
| PL-12 | 16 | 10 (62.5) | 4 (25) | 13 (81.25) | 8 (50) | 5 (31.25) | 1 (6.25) | 4 (25) | 5 (31.25) | 6 (37.5) | 4 (25) |
| OJ | 6 | 4 (66.6) | 2 (33.3) | 3 (50) | 6 (100) | 4 (66.6) | 1 (16.6) | 2 (33.3) | 4 (66.6) | 3 (50) | 3 (50) |
| EJ | 3 | 1 (33.3) | 0 | 3 (100) | 1 (33.3) | 2 (66.6) | 0 | 0 | 0 | 1 (33.3) | 1 (33.3) |
In 6 patients the coexistence of at least one ASA was found. Anti-ARS – anti-aminoacyl tRNA synthetase, ASA – antisynthetase antibodies, CK – creatine kinase, CRP – C-reactive protein, Jo-1 – anti-histidyl tRNA synthetase, PL-7 – anti-threonyl tRNA synthetase, PL-12 – anti-alanyl tRNA synthetase, OJ – anti-isoleucyl tRNA synthetase, EJ – anti-glycyl tRNA synthetase.
Discussion
The frequency of ASA among myositis-specific antibodies is 20–40% (36% in the present group). The incidence of each ASA in the study group coincided with data reported in the literature [3, 4, 9, 10].
Anti-Jo-1 antibodies were the most frequently observed ASA, followed by PL-12, PL-7, OJ and EJ antibodies. A previous large cohort of US and Japanese patients with 6 major ASA showed that the frequencies of anti-Jo-1 antibodies in these populations were 60% and 36%, respectively, anti-PL-7 antibodies were 12% and 18% and anti-OJ 2.5% and 4.8% [11, 12].
Targoff et al. [6] suggested that various ASA seem to be mutually exclusive, so an individual patient does not produce more than one, but in a small percentage of patients the coexistence of at least one ASA can be present (10% in our group).
The clinical manifestations associated with each ASA autoantibody are not identical [11–13]. It has been reported that anti-Jo-1 autoantibodies are closely associated with PM, but anti-OJ and anti-PL-12 have been more closely associated with DM skin lesions and are strongly associated with ILD [14–16].
Our data gave opposite results, as Jo-1 antibodies were associated with DM rather than PM, OJ with DM, but PL-12 with PM overlap syndrome with RA and SLE. It is important to highlight that our group was small, and reliable statistics cannot be obtained.
Arthralgia, muscle weakness and ILD were the most prevalent clinical signs associated with ASA. Our data suggest that ILD was more common in patients with the presence of OJ and PL-7, arthralgia with EJ and PL-12, and skin changes with EJ and OJ antibodies, respectively. Kalluri et al. [17] found that anti-PL12 were strongly associated with the presence of ILD, but less so with myositis and arthritis. Myositis was mainly present in patients with presence of OJ, PL-12 and PL-7 antibodies rather than Jo-1. It is worth mentioning that especially OJ antibodies can be associated with severe muscle involvement [18].
Skin involvement was a significant symptom in patients presenting Jo-1, OJ, EJ antibodies, while fever appeared primarily in patients with non-Jo-1 antibodies, i.e. PL-7, OJ, EJ, as other researchers have already observed [3]. It should be noted that only 61.5% of all cases of fever were accompanied by an increase in C-reactive protein.
A study of 166 Japanese ASA-positive patients revealed further clinical differences between the non-Jo-1 subtypes. Muscle weakness was found to be more pronounced in the anti-EJ and anti-PL7-positive patients compared with patients with anti-PL12 or anti-OJ autoantibodies. Arthritis was less common in the antiOJ-positive patients. DM rash was found to be more commonly associated with anti-EJ, anti-PL12 and antiPL7. ILD was frequently found in patients with each of the non-Jo-1 ASA [12].
It is worth mentioning that anti-Jo-1 and other ASA, such as anti-PL-12, have been observed in some patients with ILD who lack evidence of myositis [19, 20].
Although ASA belongs to MSAs, their presence is also found in the serum of patients diagnosed with non-IIM systemic connective tissue diseases. The 14 patients in the analyzed group were diagnosed with other connective tissue diseases. The role of their presence should be established.
Conclusions
The clinical presentation of patients with the presence of ASA is varied. Their presence indicates not only IIM, but also non-specifically other disease entities. These patients require observation for the development of IIM and ILD.
Footnotes
The authors declare no conflict of interest.
References
- 1.Lundberg IE, Tjarnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. Arthritis Rheumatol. 2017;69:2271–2282. doi: 10.1002/art.40320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leclair V, Lundberg IE. New Myositis Classification Criteria – What We Have Learned Since Bohan and Peter. Curr Rheumatol Rep. 2018;20:18. doi: 10.1007/s11926-018-0726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lega JC, Fabien N, Reynaud Q, et al. The clinical phenotype associated with myositis-specific and associated autoantibodies: A meta-analysis revisiting the so-called antisynthetase syndrome. Autoimm Rev. 2014;13:883–891. doi: 10.1016/j.autrev.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez C, Bardin N, De Paula AM, et al. Correlation of clinicoserologic and pathologic classifications of inflammatory myopathies: study of 178 cases and guidelines for diagnosis. Medicine (Baltimore) 2013;92:15–24. doi: 10.1097/MD.0b013e31827ebba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariampillai K, Granger B, Amelin D, et al. Development of a New Classification System for Idiopathic Inflammatory Myopathies Based on Clinical Manifestations and Myositis-Specific Autoantibodies. JAMA Neurol. 2018;75:1528–1537. doi: 10.1001/jamaneurol.2018.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Targoff IN. Laboratory testing in the diagnosis and management of idiopathic inflammatory myopathies. Rheum Dis Clin North Am. 2002;28:859–890. doi: 10.1016/s0889-857x(02)00032-7. [DOI] [PubMed] [Google Scholar]
- 7.Katzap E, Barilla-LaBarca ML, Marder G. Antisynthetase Syndrome. Curr Rheumatol Rep. 2011;13:175–181. doi: 10.1007/s11926-011-0176-8. [DOI] [PubMed] [Google Scholar]
- 8.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]; Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 9.Cruellas MG, Viana Vdos S, Levy-Neto M, et al. Myositis-specific and myositis-associated autoantibody profiles and their clinical associations in a large series of patients with polymyositis and dermatomyositis. Clinics (Sao Paulo) 2013;68:909–914. doi: 10.6061/clinics/2013(07)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghirardello A, Borella E, Beggio M, et al. Myositis autoantibodies and clinical phenotypes. Auto Immun Highlights. 2014;5:69–75. doi: 10.1007/s13317-014-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal R, Cassidy E, Fertig N, et al. Patients with non-Jo-1 anti-tRNA synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis. 2014;73:227–232. doi: 10.1136/annrheumdis-2012-201800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamaguchi Y, Fujimoto M, Matsushita T, et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One. 2013;8:e60442. doi: 10.1371/journal.pone.0060442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hervier B, Devilliers H, Stanciu R, et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun Rev. 2012;12:210–217. doi: 10.1016/j.autrev.2012.06.006. Epub 2012 Jul 5. [DOI] [PubMed] [Google Scholar]
- 14.Hirakata M, Suwa A, Takada T, et al. Clinical and immunogenetic features of patients with autoantibodies to asparaginyl-transfer RNA synthetase. Arthritis Rheum. 2007;56:1295–1303. doi: 10.10002/art.22506. [DOI] [PubMed] [Google Scholar]
- 15.Matsushita T, Hasegawa M, Fujimoto M, et al. Clinical evaluation of anti-aminoacyl-tRNA synthetase antibodies in Jap-anese patients with dermatomyositis. J Rheumatol. 2007;34:1012–1018. [PubMed] [Google Scholar]
- 16.Sato S, Kuwana M, Hirakata M. Clinical characteristics of Japanese patients with anti-OJ (anti-isoleucyl-tRNA synthetase) autoantibodies. Rheumatology (Oxford) 2007;46:842–845. doi: 10.1093/rheumatology/kel435. [DOI] [PubMed] [Google Scholar]
- 17.Kalluri M, Sahn SA, Oddis CV, et al. Clinical profile of anti-PL-12 autoantibody. Cohort study and review of the literature. Chest. 2009;135:1550–1556. doi: 10.1378/chest.08-2233. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi E, Uruha A, Suzuki S, et al. Skeletal Muscle Involvement in Antisynthetase Syndrome. JAMA Neurol. 2017;74:992–999. doi: 10.1001/jamaneurol.2017.0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshifuji H, Fujii T, Kobayashi S, et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity. 2006;39:233–241. doi: 10.1080/08916930600622884. [DOI] [PubMed] [Google Scholar]
- 20.Hozumi H, Enomoto N, Kono M, et al. Prognostic significance of anti-aminoacyl-tRNA Synthetase Antibodies in Polymyositis/Dermatomyositis-Associated interstitial Lung Disease: a Retrospective Case Control Study. PLoS One. 2015;10:e0120313. doi: 10.1371/journal.pone.0120313. [DOI] [PMC free article] [PubMed] [Google Scholar]