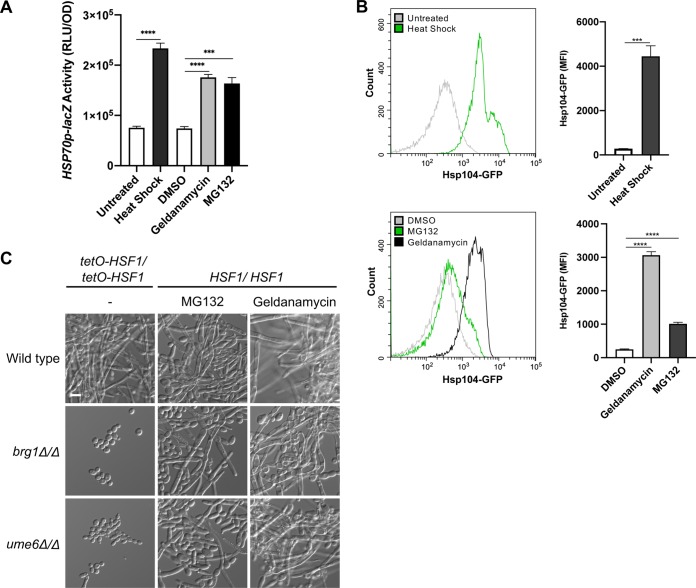
FIG 4.
Proteasome inhibition overwhelms Hsp90’s functional capacity. (A) Induction of the heat shock response was monitored using an HSP70p-lacZ reporter strain. β-Galactosidase reporter activity was measured in relative luminescence units (RLU) after incubation of cells in YPD medium with shaking at 30°C for 5 h with or without 10 μM geldanamycin, 40 μM MG132, or 0.2% DMSO as a vehicle control. Heat shock was induced by growth at 42°C for 1 h. Each bar depicts the mean of technical triplicates, and all data were normalized to the OD600. Error bars display standard deviations. ***, P ≤ 0.001; ****, P ≤ 0.0001. (B) Activation of the heat shock response was monitored by measuring the induction of Hsp104-GFP protein expression. Mean GFP fluorescence intensities (MFI) of ∼20,000 cells were measured by flow cytometry after incubation of cells under the same growth conditions as described above. Representative flow cytometry histograms depict green fluorescence of gated events. In the quantification of the flow cytometry data, each bar depicts the mean MFI of technical triplicates. Error bars display standard deviations. ***, P ≤ 0.001; ****, P ≤ 0.0001. (C) Brg1 and Ume6 are dispensable for filamentation in response to proteasome and Hsp90 inhibition but are required for filamentation in response to HSF1 overexpression. Cells were grown with 200 μM MG132 or 5 μM geldanamycin for 24 h at 30°C under static conditions. Overexpression of HSF1 from the tetO promoter was achieved by growing cells in the absence of DOX for 24 h at 30°C under static conditions. Scale bar, 10 μm.