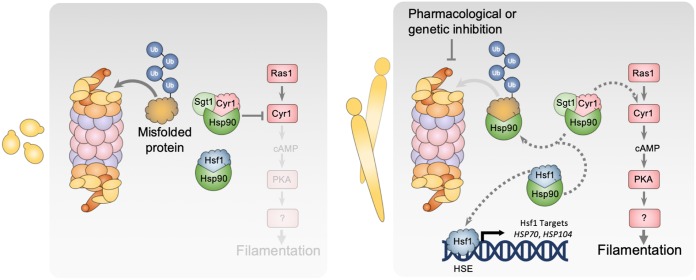
FIG 6.
Model depicting the role of the proteasome in governing morphogenesis. Hsp90 and its cochaperone Sgt1 physically interact with Cyr1, keeping it in an inactive conformation, repressing the cAMP-PKA signaling cascade. Hsp90 also binds Hsf1, which represses Hsf1 function. Inhibition of the proteasome leads to an accumulation of ubiquitinated, misfolded proteins that overwhelm the functional capacity of Hsp90, thereby relieving the repression on Hsf1 and the cAMP-PKA pathway, inducing the HSR and morphogenesis, respectively. The proteasome could also affect the cAMP-PKA pathway through additional mechanisms that are independent of Hsp90.