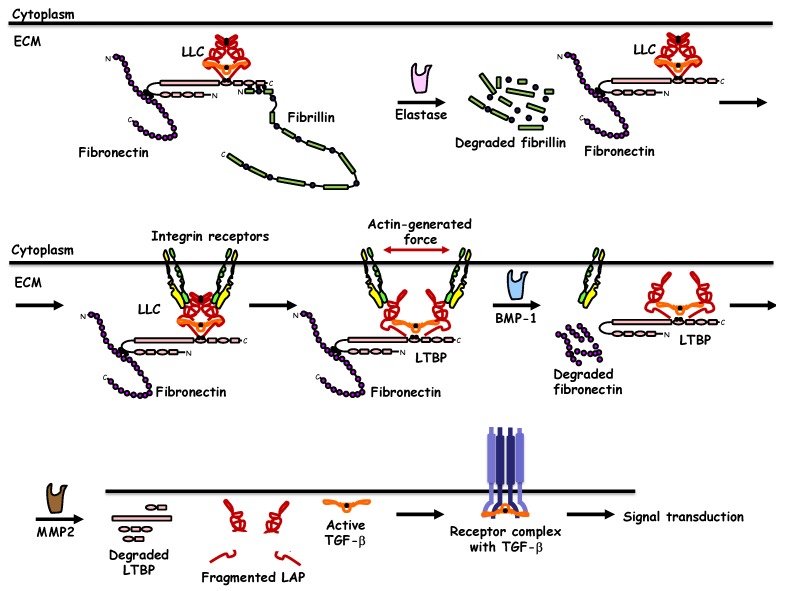
Figure 2.
Activation of latent TGF-β. A sequence of biochemical events is shown from the top left to the bottom right, guided by black arrows. The large latent complex of TGF-β (LLC) deposited to the ECM via crosslinking to fibronectin and fibrillin is shown. Elastase proteolytically cleaves fibrillin. Integrin receptors on the plasma membrane associate with the RGD peptides (not shown) of the TGF-β prodomain. Integrin heterodimers of α- and β- integrin chains are shown in different color. Integrins, via their association to the actin cytoskeleton (not shown) exert force and change the conformation of the LLC prodomain, initiating the mature TGF-β release process. BMP-1 proteolytically cleaves fibronectin and MMP2 cleaves LTBP and the prodomain of TGF-β, generating fragmented prodomain, i.e., latency associated peptide (LAP), and releasing mature TGF-β. Active TGF-β associates with the signaling type II and type I receptors and initiates signal transduction. The role of coreceptors in ligand presentation is not shown.