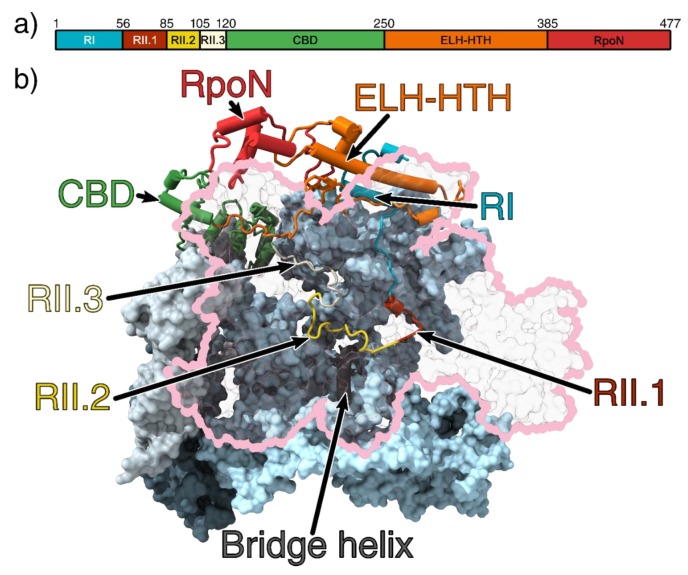
Figure 1.
(a) Domain organisation of σ54. (b) The 3.8 Å crystal structure of the RNAP-σ54 holoenzyme (PDB ID: 5NWT) showing σ54 inside the RNAP cleft. Region I and an extra-long α-helix followed by a helix-turn-helix (ELH-HTH) sit outside the cleft to form a barrier to block DNA entry, whilst the core-binding domain (CBD) blocks the RNA exit channel. The β subunit is outlined in pink and transparent for clarity [6]. The catalytic site is made up of the β and β’ cleft (pink and light blue, respectively) and are stabilised by the α1 and α2 (light and dark grey, respectively) homodimers and ω subunit (obscured by the β’ subunit) [20,21]. Region I (RI) is coloured cyan; ELH-HTH, orange; RpoN, red; CBD, green; Region 2.1 (RII.1), crimson; RII.2, yellow; and RII.3, white. All figures rendered in ChimeraX [22].