Figure 3.
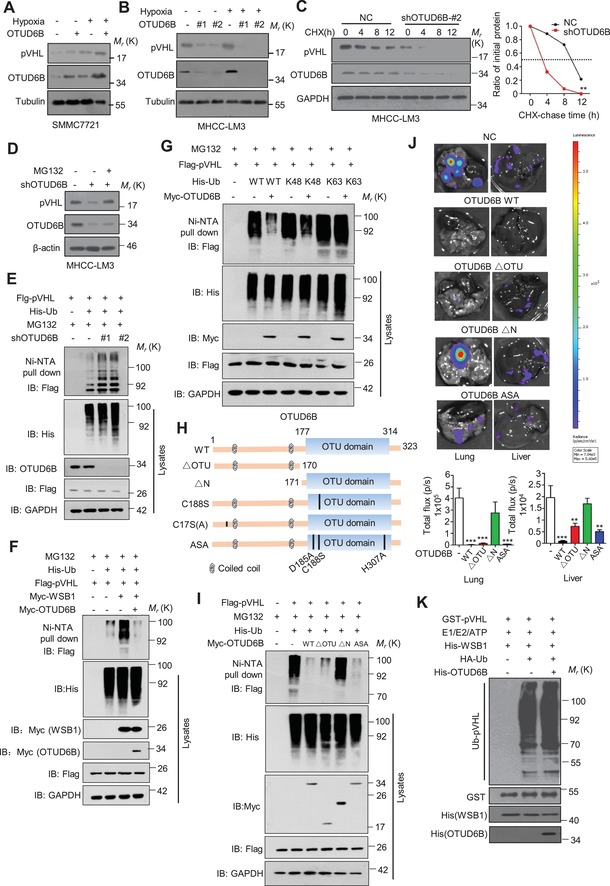
OTUD6B stabilizes pVHL in an enzyme‐independent manner. A) Immunoblotting of pVHL in SMMC7721 cells with stable OTUD6B overexpression. B) Immunoblotting of pVHL in MHCC‐LM3 cells transfected with indicated shRNA. C) HCC cells were treated with 10 µg mL−1 cycloheximide (CHX), and collected at the indicated times for western blot. Quantification of pVHL levels relative to GAPDH is shown. Results are shown as mean ± s.d. n = 3 independent experiments. ∗∗ P < 0.01, two‐way ANOVA test. D) Immunoblotting of pVHL in MHCC‐LM3 cells with shOTUD6B with or without MG132 treatment. E–G,I) His‐Ub was cotransfected together with indicated shRNA into HEK293 cells. Cells were treated with MG132 for 8 h before collection. Then His‐Ub was pulled down by Ni‐NTA and immunoblotted with anti‐flag antibody. H) Overview of the structures of OTUD6B wild type and different truncates. J) 1×106 Luciferase‐expressing SMMC7721 HCC cells with stably overexpressing indicated constructs were injected into nude mice by tail vein. The mice were euthanized 8 weeks later by a cervical dislocation. Lung (left) and liver (right) tissues were isolated for analysis of IVIS imaging. Results are shown as mean ± s.d. n = 5 independent experiments. ** P < 0.01, *** P < 0.001 Student's t test. K) Cell‐free pVHL ubiquitylation assay. The purified GST‐pVHL proteins were incubated with His‐WSB1 as E3, commercial E1, E2, and HA‐Ub for 2 h at 37 °C. The mixtures were subjected to GST pull‐down and western blot with anti‐HA antibody.
