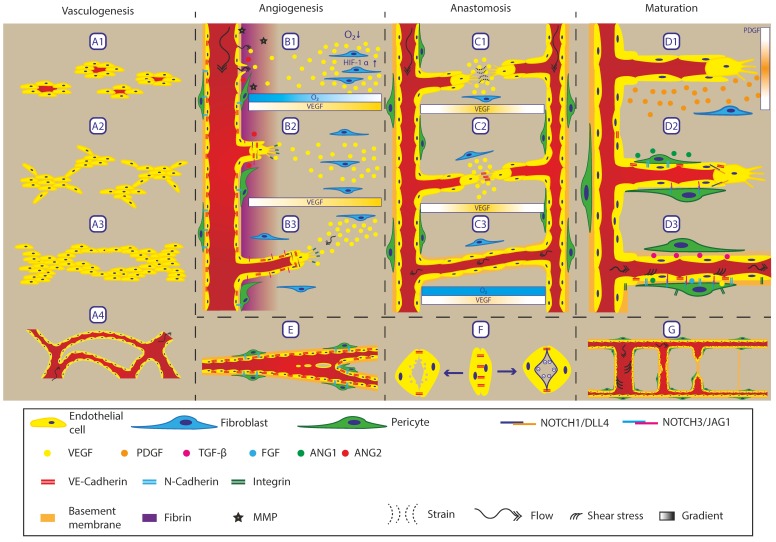
Figure 2.
Overview of processes involving remodelling of the vasculature. Vasculogenesis is the process of forming de novo vasculature. A1 The process starts with endothelial precursor cells scattered throughout the tissue. A2 These precursor cells form clumps or islands of endothelial cells, A3 which grow and form a primitive network structure. A4 Upon full connection, the network opens up for perfusion, forming a primitive plexus. Angiogenesis is the process of forming new vasculature from an existing vessel. B1 If oxygen concentration decreases, cells start to express VEGF via HIF-1α signalling which creates a gradient. When the vessel wall senses the increase in VEGF, the VE-Cadherin connections between endothelial cells loosen up, resulting in leakage of fibrin from the blood plasma into the surrounding matrix. Together with the expression and activation of MMPs, the matrix is remodelled to form a temporary matrix suitable for migration and sprouting. Sprouting endothelial cells release ANG2 upon which pericytes detach from the endothelial cells by disconnecting the N-Cadherin junctions. B2 The initial VEGF gradient results in the selection of tip cells, which use integrins to migrate through the matrix and express DLL4 to inhibit neighbouring cells, adopting a tip cell phenotype via Notch1. Via this Notch signalling cascade, a single tip cell is maintained followed by stalk cells expressing Jagged1. B3 Sprouts follow a gradient of VEGF, Semaphorins and Ephrins. Besides chemotactic migration, sprouts also migrate into the direction of interstitial flow, acting as a mechanical guidance. Due to Notch signalling within the sprout, following stalk cells stay connected to the tip cells, forming a continuous train of cells which opens up. E Besides sprouting, intussusception (or splitting angiogenesis) is also a method for forming new blood vessels. This process involves opposing endothelial cells making contact through the lumen of the blood vessel. After this, the vessel wall is remodelled until it is fully developed, resulting in a tissue pillar through the blood vessel. If this process is further promoted, a complete vessel can be split in two smaller vessels or bifurcations can be progressed into the vessel. C1 Anastomosis is guided via gradients, resulting in chemotaxis. Besides this gradient, the tip cells pull on the matrix to be able to migrate forward. This pulling results in fibres being strained between the two tip cells, forming a strain gradient that forms a guide for the tip cells to migrate towards each other and anastomose. C2 Upon contact between two tip cells, the filopodia form a connection via VE-Cadherin, stabilising the connection. This process is guided by myeloid cells expressing Notch1. C3 When the lumen of both sprouts are connected, the oxygen and nutrient levels increase, again reducing the expression of VEGF. F The formation of lumens can happen by two possible mechanisms. Left: Vacuoles are released from the endothelial cells towards the basal side of the forming lumen. This opens up a central cavity that can be perfused upon connection. Right: Glycoproteins that are negatively charged are present on the membrane at the basal side. Based on charge repulsion, the two membranes move away from each other, forming a lumen. Maturation: D1 during angiogenesis, sprouting endothelial cells express PDGF, which recruits pericytes to stabilise the vessel wall. The expression of PDGF forms a chemotactic gradient for fibroblasts to migrate towards the sprout and adopt a pericytes phenotype. D2 When fibroblasts connect to the sprouts, they adopt a pericyte phenotype. Based on this switch, N-Cadherin is expressed, connecting the pericytes to the endothelial cells and stabilising the vessel wall. Expression of ANG1 by the pericytes results in clustering at the cell–cell junctions to increase vessel tightness and results in endothelial cell quiescence. D3 Top: Expression of TGF-promotes ECM production and proliferation by pericytes as well as inducing a pericyte phenotype. Bottom: Fully stable and mature vessel wall. Pericytes adopt a phalanx state around the endothelial lumen. Low concentrations of VEGF, ANG1, and FGF are required for survival and stabilisation. Notch-3-JAG-1 signalling is required for survival and so are shear stresses, resulting from flow, acting on the endothelial cells. Endothelial cells are tightly connected together via VE-Cadherin and to pericytes via N-Cadherin; both endothelial cells and pericytes connect to the ECM via integrins. G Regression of vessels also occurs when perfusion is no longer required. The process starts by a reduced flow through the vessel resulting in a lower shear stress level. This low shear stress acts as a cue for endothelial cells to retract from the vessel and to close off the lumen. After complete retraction of the vessel, only the basement membrane is left, which will be remodelled over time to form a normal tissue ECM. Image inspired by [9,10,11].