Figure 11.
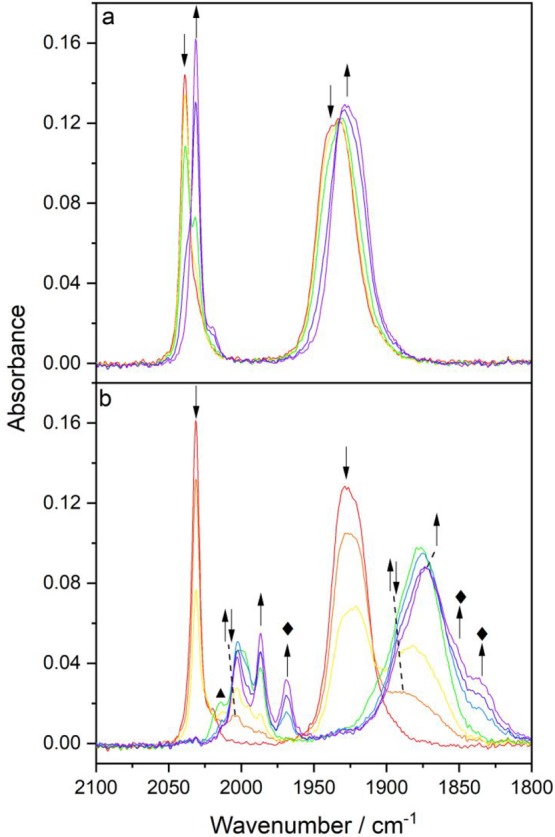
Cathodic IR spectroelectrochemistry of [Re(3,3′-DHBPY)(CO)3PrCN]+ in PrCN/Bu4NPF6 at 298 K. (a) 1e– reduction of the parent complex (↓) at R1 producing deprotonated [Re(3,3′-DHBPY-H+)(CO)3(PrCN)] (↑). (b) Subsequent reduction of [Re(3,3′-DHBPY-H+)(CO)3(PrCN)] (↓) at R2′ to give initially the 2e–-reduced ETC-species [Re(3,3′-DHBPY)(CO)3(PrCN)] (↑↓), which further converts cathodically to the double-deprotonated and 2e–-reduced 6-coordinate species, [Re(3,3′-DHBPY-2H+)(CO)3(PrCN)]3–, denoted by ⧫, in redox equilibrium with its 1e–-oxidized radical form, [Re(3,3′-DHBPY-2H+)(CO)3(PrCN)]2– (↑). The label ▲ denotes an unassigned reduced intermediate.
