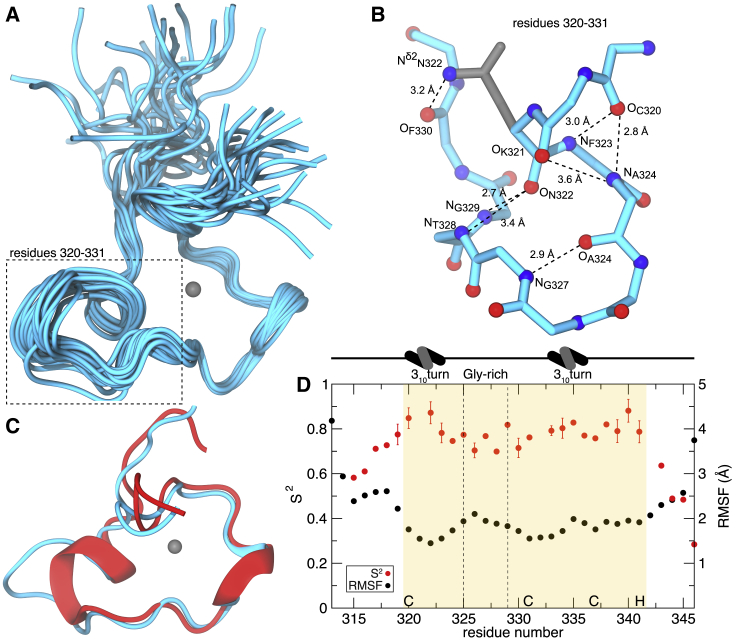
Figure 3.
The solution structure of MEX-5 ZF2. (A) The best 20 structures superimposed on backbone heavy atoms in ordered regions of the protein are shown. The zinc cation is represented as a gray sphere. (B) Hydrogen bonding within the Cys320-Cys331 region of MEX-5 ZF2 is shown. The backbone structure is depicted in cyan, oxygen atoms in red, nitrogen atoms in blue, and the side chain of Asn322 in gray. The atoms forming H-bonds are indicated in the figure. (C) A comparison of the backbone structure between TIS11d ZF2 and MEX-5 ZF2 is given. The lowest-energy structures of the ZF2 of TIS11d (red) and MEX-5 (cyan) are shown superimposed. Zinc is represented as a gray sphere. The α-helices are represented as ribbons. (D) The measure of the backbone flexibility within MEX-5 ZF2 is shown. S2 order parameters are depicted for each residue in red (bars represent the errors of the fit), and the root mean-square fluctuation within the best 20 structures are in black. The zinc finger is highlighted in the yellow box, and the Zn2+ coordinating residues, CCCH, are indicated on the x axis. The glycine-rich loop is highlighted by two dotted lines, and 310 turns are indicated at the top. To see this figure in color, go online.