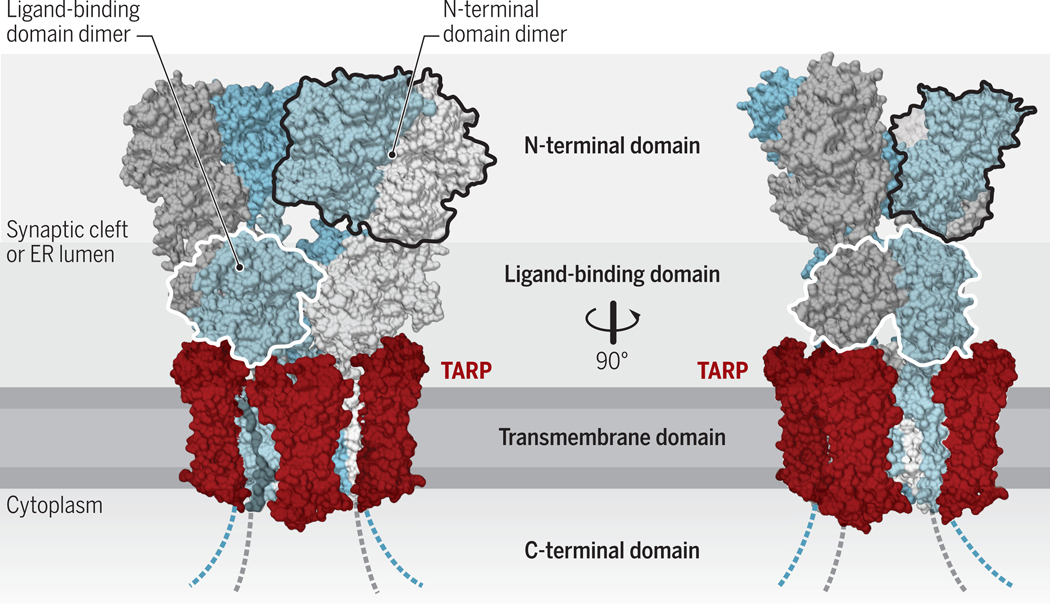
Figure 1. Structural architecture of AMPARs.
AMPARs are formed by four subunits, which are conformationally (and functionally) distinct (‘pore-proximal’ subunits are in grey and ‘pore-distal subunits’ in blue). These subunits consist of an extracellular N-terminal domain, the ligand binding domain, an integral membrane domain, and an intracellular C-terminus domain, and form tetrameric receptors (chains A-D). The large extracellular region faces the ER-lumen during receptor biogenesis and ultimately projects into the synaptic cleft. The transmembrane AMPAR regulatory proteins (TARPs) interact with the receptor at up to four positions around the transmembrane domain (two non-equivalent positions indicated in red; structure reproduced from PDB:5WEO). Credit: Adapted by A. Kitterman/Science Signaling