Fig.6.
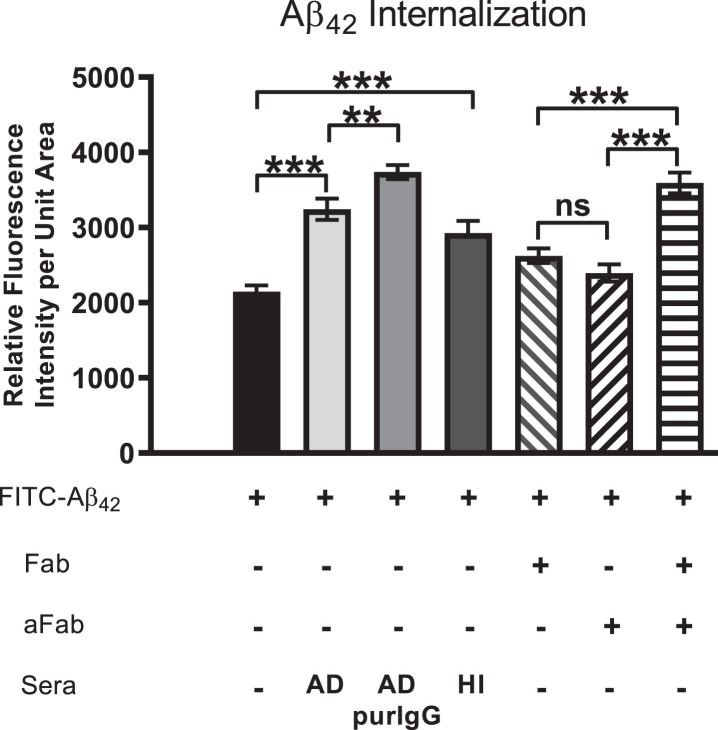
Aβ42 internalization is driven primarily by IgG cross-linking on neuronal surfaces. Cells were first treated with FITC-Aβ42 to establish a baseline level of Aβ42 internalization in the absence of other factors. Aβ42 was then co-administered with AD serum, purified IgG from AD sera, heat-inactivated (HI) AD sera (to disable binding of complement protein), monovalent F(ab) IgG fragments or monovalent F(ab) fragments co-treated with anti-F(ab) fragment secondary antibody (aFab). The latter was used to restore bivalency and thereby autoantibody cross-linking. In cells treated with monovalent F(ab) fragments (incapable of cross-linking) in conjunction with FITC-Aβ42, Aβ42 internalization was reduced compared to those treated with AD serum, purified IgG from AD serum or heat-inactivated serum. However, when media containing monovalent F(ab) fragments was supplemented with antibodies directed against F(ab) fragments to restore cross-linking abilities, the Aβ42 internalization rate was increased to a level comparable to that of purified IgG from AD serum. Fab, monovalent F(ab) fragment; aFab, anti-F(ab) fragment secondary antibody; AD, Alzheimer’s disease sera; AD purIgG, IgG purified from AD sera; HI, heat-inactivated; ns, non-significant; **p < 0.01; ***p < 0.001.