Fig.8.
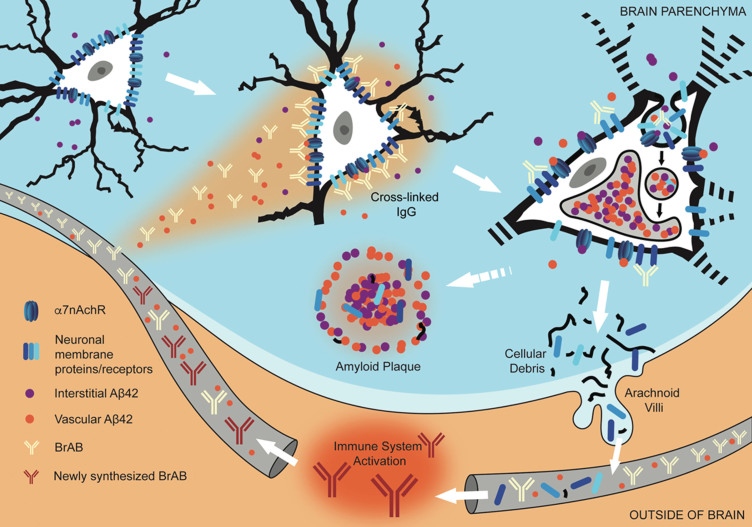
Interaction of extravasated BrABs with NSPs triggers endocytosis and results in intraneuronal accumulation of Aβ42 and depletion of NSPs from cell surfaces. Under conditions of BBB compromise, blood-borne Aβ42 and brain-reactive autoantibodies flood into the brain parenchyma. Some interact with cognate neuronal surface proteins (including receptors; NSPs), while Aβ42 binds to α7nAChRs with picomolar affinity. Bivalent autoantibodies cross-link their targets on neuronal surfaces, which can trigger endocytosis and internalization of cross-linked NSPs and surface-bound Aβ42-α7nAChR complexes. Within the acidic environment of endosomes and lysosomes, Aβ42 forms aggregates which are non-degradable and tend to accumulate. Over time, chronic leak of autoantibodies into the brain tissue results in continual internalization of Aβ42 within neurons, which interferes with their proper functioning and leads to failure to maintain their dendritic trees and associated synapses. Death and lysis of these Aβ42-overburdened neurons contributes to the formation of amyloid plaques (APs). This cell death is also a source of neuronal debris that finds its way into cerebrospinal fluid and, via arachnoid villi, into the general circulation, where it triggers an immune response leading to production of autoantibodies directed against such neuronal debris. Under conditions of increased BBB permeability, these are able to access cell surface-bound targets in the brain and thus perpetuate the engine that drives the observed pathology.