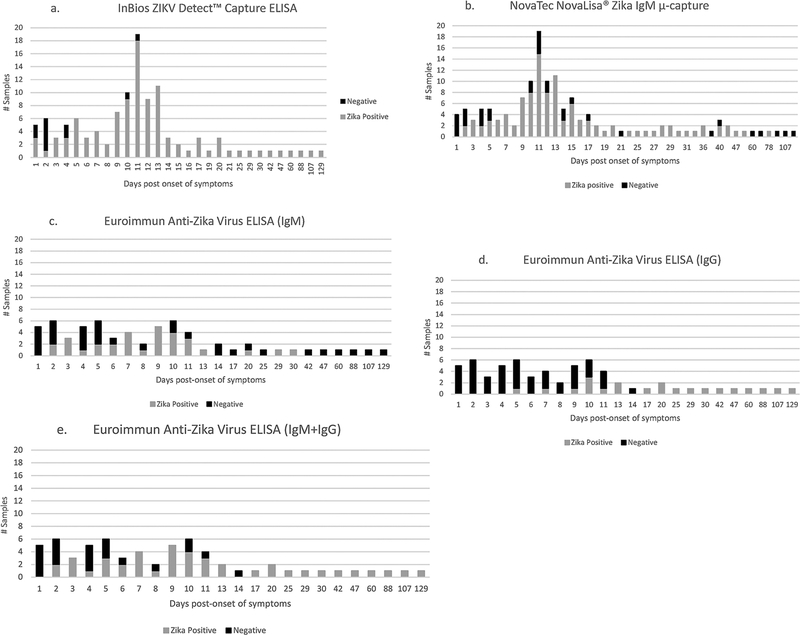
Fig. 1.
(a–e) Show the relationship between days post-onset of symptoms (DPO) and kit results for samples that tested ZIKV IgM-positive using reference methods. Numbers of samples tested at each time point are listed on the y-axis. Results in Fig. 1a for InBios ZIKV Detect™ IgM Capture ELISA were generated using serum Panels 1 and 2; results in Fig. 1b. for NovaTec NovaLisa® Zika Virus IgM μ-capture ELISA were generated using Panels 1, 2 and 3, where equivocals were classified as Zika Positive; results for Euroimmun Anti-Zika Virus ELISA IgM and IgG (Fig. 1c-e) were generated using Panel 1, where borderlines were classified as Zika Positive.