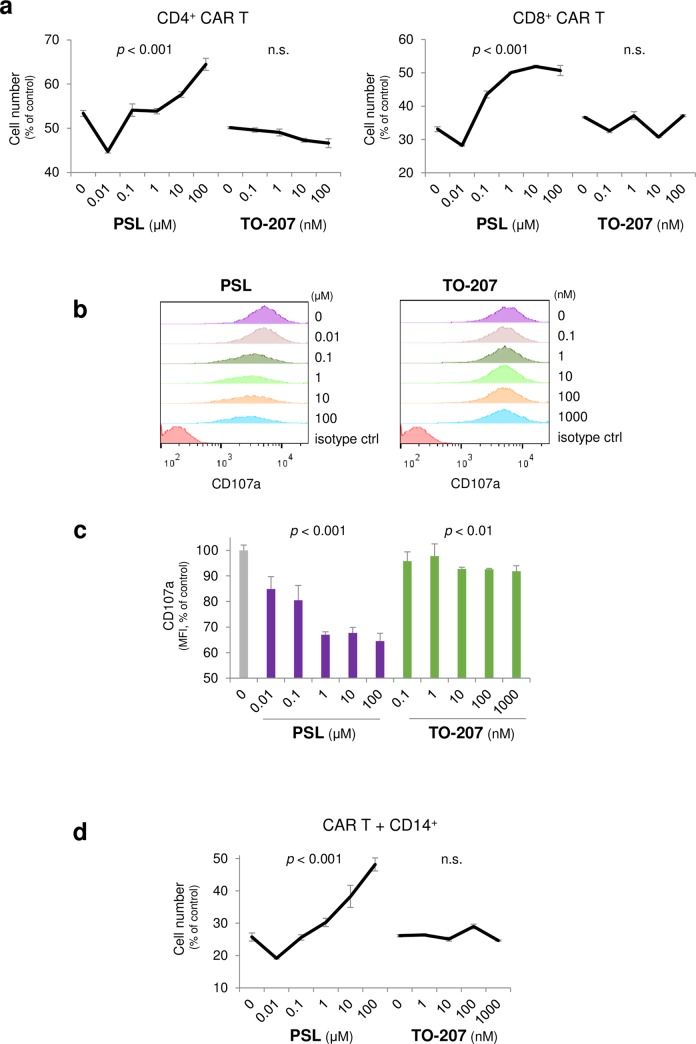
Fig 5. Advantageous pharmacological effects of TO-207 from steroid on cytotoxic effect of activated CAR T cells in the co-culture model.
A) Effects of PSL and TO-207 on the cytotoxicity of CAR T cells. K562/CD19/fLucEGFP cells (3 × 103) were co-cultured with CD4+ or CD8+ CAR T cells (1.5 × 104), and treated with different concentrations of PSL and TO-207. Viable target cells were quantified using the luciferase assay after 72 h of co-culture. Values were normalized to viable K562/CD19/fLucEGFP cells cultured alone. The error bars represent SDs from three independent experiments. B) Effects of PSL and TO-207 on CAR T cell degranulation. K562/CD19 cells (5 × 105) and CAR T cells (5 × 105) were co-cultured in 500 μl T-cell expansion medium in the absence or presence of PSL or TO-207. Following 68 h of co-culture, monensin (2 μM) and APC–anti-human CD107a antibody were added. Cells were incubated for an additional 4 h, and membrane expression of CD107a was determined by flow cytometry. Representative data of four independent experiments are shown. C) Mean fluorescence intensity (MFI) of samples in Fig 5B. The error bars represent SEs from four independent experiments. D) Sustained cytotoxicity of CAR T cells following TO-207 treatment. K562/CD19/fLucEGFP cells (1 × 104), CAR T cells (5 × 104), and CD14+ cells (5 × 104) were co-cultured in the absence or presence of PSL or TO-207 for 72 h, and viable target cells were quantified by the luciferase assay. Values were normalized to the well containing K562/CD19/fLucEGFP cells alone. The error bars represent SDs from three independent experiments. The linear dose-response relationship was assessed using log-transformed dose values (to the base 10) in a mixed model, in which the zero dose was replaced by the log (minimal dose) - 1. P < 0.05 was considered statistically significant. n.s.: not significant.