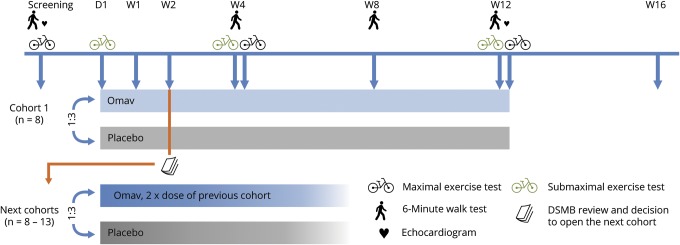
Figure 2. Design and visit schedule in a randomized double-blind dose-escalation study on omaveloxolone in mitochondrial myopathy.
Participants with mitochondrial myopathy were included in cohorts (8–13) and randomized 3:1 to omaveloxolone (Omav) or placebo treatment for 12 weeks. After 2 weeks of treatment, a data safety monitoring board (DSMB) reviewed the safety data and decided on the opening of the next dose level cohort. Six cohorts completed the protocol with 5, 10, 20, 40, 80, and 160 mg omaveloxolone, respectively. Study visits are indicated by vertical arrows and took place on day 1 (D1) and after the indicated number of weeks (W) of treatment. Laboratory measures were collected on all visits. Week 4 and 12 visits were conducted as a 2-day visit with a 6-minute walk test and a submaximal exercise test on the first and a maximal exercise test on the second day.