Abstract
Scientific literature is reviewed supporting a “consequence of war syndrome (CWS)” in Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn soldiers. CWS constituents include chronic pain and insomnia, other physical complaints, posttraumatic stress disorder (PTSD), anxiety, depression, and neuropsychological deficits. The foundation of CWS lies with the chronic stressors inherent to deployment and the cascade of biological events mediated and maintained by hypothalamic-pituitary-adrenal (HPA) axis dysregulation. Such dysregulation is modified by the individual’s specific experiences at war, difficulty reintegrating to post-deployment life, and the onset or exacerbation of the chronic and comorbid physical, emotional, and cognitive disorders. The circuit network between the prefrontal cortex (PFC), amygdala, and hippocampus is particularly sensitive to the consequences of war. The review’s specific conclusions are as follows: HPA axis dysregulation contributes to the chronic insomnia and hyperarousal seen in soldiers. There is considerable symptom overlap between PTSD and blast-related head injury, and it is difficult to determine the relative contributions of the two disorders to abnormal imaging studies. In some cases, traumatic brain injury (TBI) may directly precipitate PTSD symptoms. While not intuitive, the relationship between TBI and postconcussion syndrome appears indirect and mediated through PTSD. Blast-related or conventional head injury may have little long-term impact on neuropsychological functioning; contrarily, PTSD particularly accounts for current cognitive deficits. The psychological experience of CWS includes a “war-within” where soldiers continue to battle an internalized enemy. Successful treatment of CWS entails transdisciplinary care that addresses each of the constituent disorders.
Keywords: TBI, PTSD, postconcussion syndrome, OEF/OIF/OND, HPA axis, insomnia, cognition
A Conceptual Model for the Consequences of War
The Global War on Terror (GWoT) formally began in 2001 after the September 11 attacks. The United States led an international coalition with major combat operations in Iraq and Afghanistan. These campaigns are identified as Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) and represent the longest sustained war in US history and the first fought entirely by volunteers. By 2015, approximately 2.6 million soldiers had been deployed to these combat theaters.1
A significant portion of OEF/OIF/OND soldiers experienced traumatic brain injury (TBI), and suffer from posttraumatic stress disorder (PTSD) and symptoms akin to “postconcussive syndrome” (see section below: “Traumatic Brain Injury” for description).2-4 Furthermore, these comorbid conditions interact and can result in considerable personal suffering and impairment that continues to plague soldiers and their families long after they have left the military.
Over the last decade, our work with active duty OEF/OIF/OND service members has led us to conceptualize their difficulties as a “consequence of war syndrome (CWS)” composed of a number of interconnected biopsychosocial disorders.5-7 The overarching principle is that the consequences of war manifest as continuous rather than discrete variables so that their pernicious impact is variable. The specific constituents include multiple sources of chronic pain of which headache and orthopedic are most common; chronic insomnia often since a soldier’s first deployment; other physical complaints such as vision and balance problems; some degree of PTSD symptomatology; depression and anxiety—predominately in reaction to diminished abilities and work and family difficulties—and neuropsychological deficits that usually include slowed information processing, poor attention, distractibility, and cognitive impulsivity. The combination of these constituents as well as the variable severity of each can yield many outcomes across individuals.
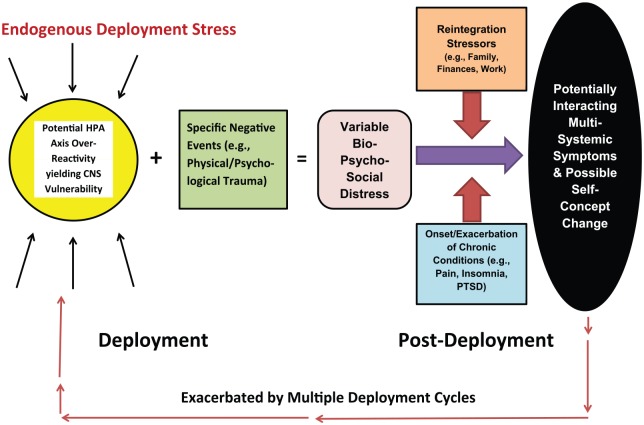
The dynamics of the CWS model entail complex interactions between the inherent stresses of deployment, difficulties reintegrating into post-deployment life, and the onset or exacerbation of the chronic, comorbid physical, emotional, and cognitive difficulties. Figure 1 schematically presents these dynamics.
Figure 1.
A proposed model of the relationship between the consequences of war and chronic post-deployment adjustment issues. CNS indicates central nervous system; HPA, hypothalamic-pituitary-adrenal; and PTSD, posttraumatic stress disorder.
Regardless of duty demands, most deployed soldiers have protracted work periods, can have disrupted sleep schedules, and/or operate with little sleep. Additional stressors include the potential for injury and death, separation from family and friends, and a complex work environment. It is likely that the deployment predicament evokes hypothalamic–pituitary–adrenal (HPA) axis over-reactivity that potentiates the neurotoxic effects of stress hormones and yields central nervous system (CNS) vulnerability. Therefore, the impact of specific deployment events such as TBI, other physical injuries, and psychological trauma may be far greater than if analog experiences occurred during a non-deployment period. Thus, it is reasonable to conclude that many soldiers return home with varying degrees of compromise.
Once home these soldiers are impacted by psychosocial stressors inherent to non-deployment life (eg, a less structured environment, adjusting to different work duties, increases in social demands from family and friends, and the resumption of household responsibilities such as financial management). There is also the potential development or exacerbation of the compounding medical conditions introduced above. Our patients consistently acknowledge that their physical, cognitive, and emotional symptoms worsen over time, likely from these conditions interacting with each other and with the psychosocial variables, as well as the effects of multiple deployments.
We will use the tenets of the CWS model to interpret the research reviewed below. Following further discussion of the role of HPA axis reactivity and insomnia in CWS dynamics, we will introduce military-related TBI and PTSD, and will present studies that investigated the effects of the two disorders on brain structure and function and those comorbid physical, emotional, and cognitive conditions common to OEF/OIF/OND soldiers and veterans. By definition, the CWS model reflects complex interactions; however, we will take steps to identify the specific contributions of HPA axis reactivity, TBI, and/or PTSD.
The Activating and Potentiating Role of Stress and HPA Axis Reactivity in CWS
As evident in Figure 1, we maintain that the foundation of CWS lies within the endogenous and exogenous stressors inherent to the deployment predicament and the cascade of biological events mediated and maintained by HPA axis reactivity across time. Such reactivity is further modified by the individual’s specific experiences at war and when they return home. As proposed in this and subsequent sections, the circuit network between the prefrontal cortex (PFC), amygdala, and hippocampus is particularly sensitive to the consequences of war.
HPA axis activation and CNS effects
In reaction to real or perceived threats, the human stress response entails the coordinated activation of the autonomic nervous (ANS) and neuroendocrine systems.8 Stress exposure causes the paraventricular nucleus (PVN) of the hypothalamus to release corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP).9 The anterior pituitary is activated by CRH and AVP and secretes adrencorticotropic hormone (ACTH) which stimulates the production of corticosteroids by the adrenal cortex.
As corticosteroids cross the blood-brain barrier they bind to the glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs).9 GRs are distributed throughout the brain, but are most common in the hypothalamic CRH neurons and pituitary corticotropes. MRs are primarily in the limbic region with the greatest concentration in the hippocampus.
Stress hormones can produce biological effects on a variety of CNS functions that support cognition, emotion, and behavior.10 McEwen recently detailed the brain’s structural and functional plasticity in response to stressful experiences.11 Reactions can include neuronal replacement, dendritic remodeling, and synapse turnover that yield neural circuitry imbalance. These dynamics can undermine cognition and decision-making, as well as produce anxiety and mood disturbance. Neural circuitry imbalance can affect systemic physiology through neuroendocrine, autonomic, immune, and metabolic mediators. Prolonged stress resulting in corticosteroid dysfunction yields widespread inflammation and contributes to chronic pain (an important CWS constitute).12 The stress of contending with these consequences (eg, anxiety, depression, chronic pain, insomnia, and cognitive difficulties—each also being linked to HPA over-reactivity) further compounds the individual’s global stress and subsequent HPA axis response.13 We maintain this is an important CWS mechanism whereby each specific condition (eg, insomnia) negatively impacts the other disorders (eg, chronic pain, depression, anxiety, cognitive deficits) which in turn exacerbate it. Such dynamics would exact a physiological cost on the soldier yielding a “high allostatic load” which can diminish resiliency, increase vulnerability, and weaken them as they attempt to cope with life’s challenges.12 Across time, this complex biopsychosocial phenomenon could persist vacillating between periods of relative rebound and “allostatic overload.”
Insomnia: a particular CWS stressor
Germain et al14 reported that deployed soldiers commonly endorse sleep disturbances including increased latency, wakefulness after sleep onset, short duration, and fragmentation. As many as 70% of service members experience chronic, clinically significant insomnia following deployment. Using the Pittsburgh Sleep Quality Index, 89% of nearly 400 OEF/OIF soldiers and veterans were classified as “poor sleepers.”15 Approximately, 45% took longer than 30 minutes to fall asleep at night, 21% slept less than 4.5 hours per-night, and 15% reported being awake for more than 15% of the night. In a sample of nearly 300 active duty soldiers, our own patients report that on average they have experienced insomnia for nearly 6 years and that they also sleep less than 5 hours-per-day.6 Chronic insomnia is also strongly correlated with war-related conditions including TBI, postconcussion syndrome, and PTSD.
The harmful effects of long-term insomnia on general health is well-established and include an increased risk of hypertension, diabetes, obesity, depression, anxiety, heart attack, and stroke.16 This may have contributed to the findings of a large prospective study which observed that despite their young average age and good pre-deployment health, soldiers evidenced a clinically significant decline in physical and mental functioning (as measured by a wide range of dependent variables subjected to covariant strategies and statistical modeling) over the course of a year-long deployment.17 Furthermore, these soldiers’ health status continued to decline after the deployment.
Growing research is establishing the negative impact of insomnia on the brain. A recent resting state functional connectivity magnetic resonance imaging (fcMRI) study revealed that persons with primary insomnia had abnormalities in intracephalic multisystem structure and neural network connection. These abnormalities were more pronounced if the individual also had depression (a core CWS constitute).18 Persons with chronic insomnia have also been shown to have a weak connection between the default mode network (DMN [see “Human Imaging Studies” for further DMN description]) and the supplemental motor area (involved in movement control such as temporal sequencing, postural control, and bimanual coordination) a known functional MRI (fMRI) marker of inattention. Furthermore, the younger the age of insomnia onset, the weaker the connection.19 Additional discussion of the effects of insomnia on the brain and cognition is presented below (see “Posttraumatic Stress Disorder”).
Insomnia as a consequence of HPA axis overactivation and hyperarousal
Non-military, healthy individuals who loose just one night of sleep have shown elevated resting cortisol release and an exaggerated cortisol response to a stressful challenge suggestive of HPA axis over-reactivity.20 In a recent review, Vargas et al21 concluded that the neurobiology of chronic insomnia arises from HPA axis dysregulation. Specifically, they propose that an abnormal ultradian cortisol rhythm is responsible for insomnia since it plays an important role in sustaining daytime wakefulness, and through a relative absence of ultradian pulses at night, permits consolidation of sleep and/or shorter nocturnal awakenings.
Basta et al22 introduced an intriguing theory that chronic insomnia is not a state of sleep loss, but a hyperarousal stress disorder that persists across day and night. Their argument is supported through a comprehensive review of research examining sleep patterns, electroencephalography (EEG) activity, polysomnographic studies, neuroimaging, cognitive function, and HPA axis and inflammation markers.
Regarding brain activity and consistent with hyperarousal, persons with insomnia present different EEG patterns before sleep onset and during sleep compared to normal sleepers.22 Immediately prior to sleep they demonstrate elevated beta wave power (associated with alertness) indicative of high-frequency EEG activity and decreased slow delta power (typical of deep dreamless sleep). During non-rapid eye movement (REM) sleep, persons with insomnia show higher levels of beta and gamma wave power (the fastest activity, associated with information processing) and decreased theta (reflecting deep relaxation) and delta power. During REM sleep (associated with heightened brain activity and dreaming) beta and alpha power increase and there is a deficit of theta and delta power; persons with insomnia also exhibit reduced alpha power (associated with relaxation) while awake.
Positron emission tomography (PET) studies reveal that during sleep persons with insomnia show elevated global cerebral metabolism consistent with hyperarousal.22 As they transit into sleep, they exhibit a smaller decline in brain activity within regions that control wakefulness. Contrarily, during wakefulness, persons with insomnia show decreased prefrontal activity that may underlie their common daytime fatigue.
Basta et al22 conclude that HPA axis reaction to physical (eg, chronic pain) and/or psychological stress underlies both the hyperarousal and disturbed sleep in persons with insomnia. CRH and cortisol cause arousal and sleeplessness in humans and animals, whereas deep sleep inhibits the stress system. Insomnia is associated with an overall 24-hour increase of ACTH and cortisol secretion; furthermore, the greatest elevations are observed in the evening and during the first half of the night. Finally the greater the degree of objective sleep disturbance, the higher the cortisol concentration.
Summary: the chronic stress of deployment
The conclusions of Vargas et al21 and Basta et al22 have particular utility for the CWS model, especially with how deployment may precipitate the syndrome. As indicated above, there are numerous obvious and subtle stressors when one goes to war, even if it does not entail direct combat exposure. Furthermore, chronic stress is associated with a variety of physical and psychiatric disorders (eg, depression, chronic pain, insomnia, PTSD, etc. [see above]) that in our population commonly originate during deployment and are then exacerbated by post-deployment/readjustment demands.
As the deployed soldier is exposed to stress, the normal HPA axis response ensues with the production and release of corticosteroids (eg, cortisone, hydrocortisone, & prednisone). While there can be some habituation of the HPA response to a repeating stressor, this is less likely with intense stressors such as the physical and psychological experiences encountered during deployment. Furthermore, even if there is some degree of habituation to one type of stress, the HPA response to a novel stressor is either unchanged or even greater; thus, there are biologic mechanisms that drive and change the HPA axis even though the feedback signal created by glucocorticoid exposure increases.23
The myriad stressors encountered by deployed service members, as well as individual (eg, possible genetic predisposition [see below: “Posttraumatic Stress Disorder”], military occupational specialty [MOS], number of deployments, pre-deployment stressors, etc) and behavioral factors (eg, diet, physical exercise, tobacco use, stress management, etc), can further contribute to a high allostatic load that maintains neuroendocrine instability. Such instability increases as the individual begins to experience the consequences of chronic glucocorticoid release. As detailed above, stress hormones can produce direct CNS changes that negatively impact cognition, emotion, and behavior.10-13 These psychological consequences can diminish how the service member copes with deployment demands, thus increasing their vulnerability to ongoing and subsequent stressors. The effects of chronic glucocorticoid exposure can also directly hinder the soldier’s physical reserves (eg, initiating insomnia, impacting blood pressure and weight management, and precipitating inflammation that can contribute to chronic pain).
That corticosteroids can elicit hyperarousal may be particularly important to the development and maintenance of CWS. As introduced above, there is an apparent link between HPA over-activation and insomnia21,22—a common and chronic disorder in OEF/OIF/OND soldiers. Essentially, an interrelationship develops between stress, HPA reactivity, the development of behavioral hyperarousal, and the onset and persistence of insomnia. Thus, the service member experiences the conjoint, negative consequences of corticosteroids and chronic insomnia, both conditions which have been shown to directly impact brain function, as well as the resulting and compounding physical, cognitive, and emotional vulnerabilities that further contribute to potential allostatic overload and which can undermine global functioning.
The second component of the CWS model presented in Figure 1 (see “A Conceptual Model for the Consequences of War”) introduces additional experiences that might interact with and exacerbate the effects of general deployment stress and HPA axis hyper-reactivity. They primarily revolve around more intense physical or psychological injuries—particularly those associated with TBI and PTSD.
That there is an entire PTSD diagnostic cluster of hyperarousal symptoms points to a possible interaction between HPA axis reactivity, subsequent behavioral hyperarousal, and the onset of the disorder in service members with a history of combat deployment. Further support for such a relationship is the strong positive correlation between the severity of PTSD symptomatology and chronic insomnia—another disorder linked to excessive HPA axis reactivity. Regarding TBI, a strong relationship has been identified between physical injury and PTSD onset, with the greatest occurrence being in soldiers whom have experienced head injuries (see “Diagnostic & Symptomatic Relationships”). A particular question is whether this relationship arises from the psychological consequences associated with an injury to the head, or if the physical dynamics of blast exposure (see below) might precipitate PTSD. In an effort toward answering such questions, as well as to further elaborate the CWS model, we direct our discussion to TBI and PTSD in OEF/OIF/OND service members.
TBI
The disorder defined
Head injury severity is primarily determined by its immediate impact on consciousness and for a relatively short period thereafter. The distinguishing factors are usually length of alteration/loss of consciousness (AOC/LOC) and posttraumatic amnesia (PTA: a period that the person is unable to remember events following the head injury).6,24 Mild head injuries (often labeled concussions) are associated with LOC up to 30 minutes, and AOC and/or PTA of 24 hours or less. Moderate head injuries with LOC between 30 minutes and 24 hours, AOC greater than 24 hours and PTA greater than 24 hours but less than 7 days. Severe head injury is defined by LOC or AOC of more than 24 hours and PTA greater than 7 days.
Regardless of cause, between 75% and 90% of civilian head injuries in the United States are mild.25 People who experience mild TBI (mTBI) commonly endorse a variety of somatic, cognitive, and emotional symptoms during the acute (ie, 1-7 days) and subacute (ie, 8-89 days) phases of recovery. While the expectation is that persons with mTBI will make a rapid and complete recovery, about 20% continue to experience three or more symptoms beyond 3 months and are said to have postconcussion syndrome.26 This group of patients often do not respond well to care and can be a point of contention between clinicians whom argue that postconcussive syndrome is primarily a psychogenic or neurogenic disorder.25
It is important to distinguish between postconcussive syndrome and “postconcussive symptoms.” Postconcussive symptoms (PCS) are those specific physical, emotional, and cognitive difficulties seen after head injury. The list of PCS usually includes headache, dizziness, blurred vision, tinnitus (ie, ringing in the ears), noise and light sensitivity, attention and memory deficits, insomnia, fatigue, irritability, and anxiety. As indicated above, postconcussive syndrome is the persistence of three or more PCS beyond 3 months.
TBI in OEF/OIF/OND veterans
TBI is frequently referred to as the “signature wound” of OEF/OIF/OND soldiers because its occurrence is said to be greater than in prior conflicts. It is estimated that 78% of OEF/OIF injuries and 40% of OIF combat deaths were from blasts explosions.27 Results from a recent large national study revealed that about 17% of OEF/OIF/OND veterans sustained TBIs.28 Most of the injuries were from blasts or other explosions; about 46% resulted in LOC, 90% AOC, and nearly 90% were classified as mild. Like civilians with mTBI, common associated features included headache (58%), memory problems (48%), sleep disturbance (44%), irritability (40%), balance problems or dizziness (29%), and light sensitivity (29%). Given that the participants were no longer active duty service members, a large portion of those with a head injury history were beyond the post-acute recovery phase and would qualify as having postconcussive syndrome.
Chapman and Diaz-Arrastia29 propose that there are important differences between military and civilian TBI: Combat operations make it more difficult to report and document head injury. Civilian head injuries are usually distinct events; military TBI often occurs within the context of a continuous mission. As indicated above, soldiers are often sleep-deprived and highly stressed which diminishes resiliency and undermines normal recovery. TBI effects can be hard to distinguish from comorbid mental health conditions (eg, anxiety, depression, PTSD). Finally, high-energy explosions may impact the brain in novel ways.
A recent study by Tsao et al30 reported that blast wave explosions are unique and can negatively impact soldiers even if they do not experience a concussion. The factor that appeared to determine symptom-onset was whether the service member was physically moved by the blast(s). If a concussion did occur, service members were significantly more likely to have five or more PCS. The probability of this increased further if they had a previous concussion. Tsao et al concluded there is a continuum of symptom severity with a history of recurrent concussion being most problematic, followed by a single concussion, blast exposure resulting in movement/injury, blast exposure without movement/injury, and finally, no concussion/blast exposure.
A similar response continuum was reported by Mac Donald et al31: soldiers with TBI showed the greatest global disability and the most severe depression, PTSD symptoms, and neurobehavioral impairment followed by the non-TBI, blast-exposed participants and the control group. In a related study, Mac Donald et al32 found that at one- and five-years after the initial evaluation 72% of blast-concussed patients demonstrated a poorer score on the Extended Glasgow Outcome Scale versus 11% for the control group. The TBI patients reported significantly poorer satisfaction with life and sleep, as well as neurobehavioral and psychiatric symptoms including PTSD and depression.
Review of these relatively few studies reveals that complex interactions between situational and head injury factors support tenets of the CWS model. Factors such as stress and sleep deprivation likely yield CNS vulnerability and lengthen the recovery time for even mild head injury. The effects of blast exposure are significant, even if concussion does not occur, and concussive or sub-concussive events interact with or contribute to other disorders including chronic insomnia, PTSD, depression and psychosocial adjustment.
Blast TBI: acute effects
Vascular and neuronal consequences
We will focus on blast explosions since they are the most common GWoT head injury and it is likely that far more soldiers have been affected by them than those who were formally diagnosed with concussion. Blast is qualitatively different from conventional head injury and is marked by extreme changes in atmospheric pressure: first there is intense pressure (“overpressure”) and then a corresponding pressure drop (“underpressure”).33 Within the cardiovascular system the blast’s kinetic energy is transferred into hydraulic energy causing “a volumetric blood surge” from the high-pressure body cavity to the low-pressure cranial cavity.34 The high-pressure from the blood surge then causes damage to tiny cerebral blood vessels and the blood-brain barrier. Additional events may include changes in the vasculature or in areas of transition between more and less dense areas of the brain, particularly at gray-white matter junctions.
Cernak35 describes additional systemic reactions to blast beyond the volumetric blood surge. These include the formation of air emboli that can seriously decrease blood flow velocity and cause tissue convulsion—likely secondary to hypoxia or anoxia; ANS activation including vagally mediated blood pressure drops that can also produce cerebral hypoxia; and that blast can activate a variety of inflammatory mechanisms including autacoid (biologic factors that act like local hormones) release that affect cellular and humoral immunity leading to brain inflammation.
Przekwas et al36 further describe how the blast specifically assaults the brain. Regardless of the primary injury mechanism, diffuse axonal injury (DAI) can occur across a wide area resulting in micro-damage that manifests as “impairment to neurofilament units of the axonal cytoskeleton, loss of membrane integrity, and Wallerian-type axonal degeneration.”
Przekwas et al36 propose that DAI is actually a secondary process and that synaptic injury represents the primary blast effect. Essentially, the blast produces stretching and shearing of synapses that disconnects neural circuitry and results in a temporary loss of neuronal communication. As a result of this primary micro-damage, several physical and neurochemical events result which can last from minutes to hours and conclude in either axonal and synaptic repair or persisting damage. Przekwas et al argue that the biomechanical micro-damage to synapses, dendritic spines and axons significantly contributes to mTBI etiology through an imbalance between post-trauma excitatory and inhibitory neuronal processes.
Epigenetic factors
A comprehensive treatment of TBI gene X environmental interactions is beyond the scope of this review; nonetheless, an introduction is warranted given the general principles of the CWS model. A study undertaken by Heinzelmann et al37 was the first to support the possibility that the blast explosions encountered by GWoT soldiers significantly alter gene activity leading to delayed neuronal recovery and contributing to chronic PCS.
While the sample consisted of only 36 soldiers (TBI = 19 & control = 17, matched on age, gender, race, PTSD, insomnia, and depression) they were assessed during deployment. Peripheral blood samples revealed differences in 34 transcripts in 29 genes.37 Specifically, the upregulated (a cell increases the quality of a cellular component [eg, RNA, protein] in reaction to an external stimulus) genes included epithelial cell transforming sequence and zinc figure proteins. These transcriptions are involved in astrocyte differentiation. Following TBI, astrocytes facilitate neuronal survival by potentiating collateral synapses, the migration of neuronal progenitor cells, and the differentiation of glial progenitor cells. Contrarily, the tensin-1 (TNS1: a gene pivotal to neuron recovery following TBI) and epidermal growth factor receptor (EGFR: a peripheral precursor vascular endothelial growth factor that has been shown to be a neuro-protector following TBI) genes were down-regulated (a cell decreases the quality of a cellular component in reaction to an external stimulus) in the TBI group. Heinzelmann et al argue that reductions in TNS1 and EGFR lead to poor neuronal repair, insufficient regeneration and contribute to the onset on chronic PCS.
Heinzelmann et al37 place their results within a wider model of blast-TBI effects that initiate over-activation of the ANS and neuroendocrine-immune system. While these epigenetic findings further complicate the deployment matrix, they may provide additional clarification of CWS pathways. As discussed above, blasts effects are likely magnified by inherent deployment stressors, the ensuring HPA over-reactivity, and its impact across multiple biological systems. Blasts themselves would further contribute to allostatic overload. In addition, and as will be discussed below, there is support that blast-TBI may precipitate PTSD in some soldiers and that it, and not head injury, accounts for the protraction of PCS and neuropsychological deficits.
PTSD
The disorder defined
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition describes PTSD as a psychiatric disorder arising from direct or indirect exposure to events that may lead to serious injury, sexual violence, or death. PTSD consists of four symptom clusters38: (1) A variety of re-experiencing phenomena that include intrusive and involuntary thoughts or images, distressing dreams, dissociative reactions such as flashbacks (ie, remembrance of a traumatic event as if it were happening and which can include experiences across the five senses), and excessive emotional and physiological reaction to external environmental or internal mental cues that are associated with traumatic experiences. Persons with PTSD will usually engage in (2) avoidant behaviors to evade internal (eg, thoughts or memories) or environmental (eg, locations, conversations, movies and TV) reminders of traumatic events. As a result, the individual becomes isolated from others. PTSD is further associated with (3) cognitive distortions, memory deficits (ie, inability to remember aspects of traumatic experiences), and negative emotional states. PTSD sufferers are commonly fearful, angry, guilty, or shameful. They also hold negative beliefs and expectations about themselves (eg, poor self-worth, self-blaming, that they will soon die) or the world (eg, no one can be trusted, and no one is safe), diminished interests, detached feelings toward others, and difficulty experiencing positive emotions. Finally, PTSD includes (4) heightened CNS and ANS arousal and reactivity that manifests as irritability, angry verbal and/or physical outbursts, reckless and/or self-destructive behavior, hypervigilance and exaggerated startle, poor concentration and insomnia.
PTSD in OEF/OIF/OND veterans
Epidemiological studies of GWoT soldiers yield variable findings. Across studies, it has been estimated that between 4% and 33% of soldiers who deployed to Iraq and/or Afghanistan have a current/lifetime prevalence of PTSD.39 Studies examining what makes a soldier more vulnerable to develop PTSD have also yielded mixed results, and common variables such as gender and race may play less of a role in the military/veteran population. It does appear that higher combat exposure results in more serious PTSD symptomatology that hinders the soldier’s reintegration and worsens family relations across deployments.40-42
As introduced above, OEF/OIF/OND veterans with PTSD usually experience insomnia and its severity is proportional to that of the PTSD symptoms.43 Some symptoms of insomnia such as daytime sleepiness may be worse if there is also a history of mTBI.44 Mohlenhoff et al45 proposed that the greater risk for dementia shown by persons with PTSD is related to several specific effects of chronic insomnia on the brain. These consequences include cellular damage to structures crucial to learning and memory, the accumulation of harmful amyloid proteins, and elevated inflammation, which, in turn, leads to cytokine-mediated neural toxicity and reduced neurogenesis (ie, birth of new brain cells). Insomnia has also been shown to hinder the release of acetylcholine—a neurotransmitter that plays an essential role in the brain’s ability to form and store memories (ie, “memory consolidation”).46 Finally, clinically significant relationships have been established between insomnia and cognitive impairment, especially with attention and episodic memory.47
A relationship has also been proposed between PTSD and obstructive sleep apnea (OSA) in younger OEF/OIF/OND veterans (mean age = 33.40 years, SD = 8.35).48 Logistic regressions found that PTSD severity predicted high risk for OSA even after controlling for risk factors (age, smoking, use of CNS depressants). Furthermore, blood pressure and body mass index did not increase the risk of screening positive for OSA. In our own active duty patients (mean age = 34.60 years, SD = 6.21), we have found that about 40% have been diagnosed with OSA following polysomnographic study.6 Unfortunately, only about 50% of these patients consistently uses their continuous positive airway pressure (CPAP) machines. The relationship between OSA, sleep disturbance, and cognitive difficulties is well-established.49 Further research is needed to confirm a relationship between PTSD and OSA and determine the underlying etiology.
The existing research on the clinical course of GWoT PTSD is limited. It is known from Vietnam veterans that PTSD can persist for decades and even a lifetime and that there can be remissions and relapses; furthermore, it is believed that there is a delayed subtype of PTSD that may not fully manifest until months or years after a trauma.50 Kennedy and Wang51 reported that 35% of OEF/OIF/OND veterans may develop PTSD over time.
The relatively few longitudinal studies suggest that the burden of PTSD symptoms increases over time and that about 41% of active-duty OEF/OIF soldiers who endorsed significant symptoms post-deployment showed a chronic trajectory at the 6-month mark.52 Findings consistently support that the impact of PTSD on the quality of life of OEF/OIF veterans is comparable to other war cohorts.53
PTSD: theoretical models
Neurocircuitry
Shin, Rauch, and Pitman proposed a seminal PTSD model linking the neuro-circuitry between the medial prefrontal cortex (MPC: executive functions such as the initiation, monitoring and inhibition of actions), the amygdala (a limbic structure that plays a role in the assessment of threat-related stimuli and fear-conditioning) and the hippocampus (another limbic structure important for the consolidation of information from short-term to long-term memory).54 The tenets of the neuro-circuitry model are as follows: (1) amygdala hyper-responsivity to threat-related stimuli: hyper-responsivity mediates symptoms of hyperarousal and explains the indelible quality of the emotional memory for traumatic events. (2) Reduced MPC governance over the amygdala: Inadequate MPC influence leads to deficits in extinction (ie, the emotional intensity for an experience diminishes little over time). (3) Decreased hippocampal function underlies deficits in identifying safe context and explicit memory (ie, willfully recalling information): Experiential learning is further hindered by deficits in memory consolidation.
Recently, Zotev et al55 described the role functional neural networks play in PTSD. Excessive threat-detection comes from “salience network (SN)” impairment. The predominant SN function “is to identify the most homeostatically relevant internal and/or external stimuli.”56 Important SN constituents include the amygdala, dorsal anterior cingulate cortex (contributes to cognition, emotion and chronic neuropathic pain), and the insula (involved in numerous functions including the detection of novel stimuli, the self-awareness of physiological state [“interoception”], empathy, risky decision-making, and negative emotional experiences).57
Deficits in the executive function/emotion regulation (EF/ER) system are further said to hinder persons with PTSD. The EF/ER system is composed of PFC areas including the dorsolateral, ventrolateral, and medial MPC.55 These brain regions orchestrate a wide range of higher-order cognitive and emotional functions including sustained attention, working memory, mental set-shifting, response monitoring and inhibition, information updating, temporal coding, episodic memory, situational learning, and motivation.58 Furthermore, the PFC has reciprocal connections with most cortical and subcortical structures.
Finally, Zotev et al55 maintain that functional deficiencies in contextual processing (CP) lead to the difficulties in threat discrimination commonly shown by persons with PTSD. CP allows us to extract relevant situational information in order to select the most appropriate task-specific responses, and it is deemed essential for flexible-adaptive behavior. The structures involved in CP include the hippocampus, the thalamus (located between the cortex and brain stem and involved in numerous functions including interconnected cortical, subcortical, and cerebellar circuits and plays important roles in attention, information processing speed, and memory),59 and the locus coeruleus (LC: synthesizes norepinephrine [NE] which contributes to synaptic plasticity, energy homeostasis, control of blood flow, pain modulation and motor-control60). The LC-NE system plays a substantial role in arousal, attention, and stress responses.
Genetic contributions
Epigenetic interactions have been shown to effect phenotypes (the observable result of gene X environmental interactions) across a variety of disorders. Examinations of such contributions to PTSD is a highly active research field. We offer only a cursory review of the most recent findings to further punctuate our position that the heart of consequence of war effects arise from nervous system changes. Blacker et al61 acknowledge that efforts to determine PTSD etiology and phenotypy is complicated and that findings obtained from military and civilian PTSD populations may be incompatible due to differences in age, sex, and race ratios, socioeconomic status, the incidence of TBI, frequency and severity of trauma, and intense social cohesion (a protective element for active duty soldiers that disappears after leaving the military).
Pertinent to our discussion is that as a consequence of the HPA stress response, corticosteroid receptor binding can lead to the induction or repression of the transcription of over 200 genes that are involved in a multitude of cellular processes.9 This provides a mechanism through which corticosteroids can impact the brain, initiating terminal maturation, remodeling axons, affecting cell survival, and resulting in altered neurobehavioral functioning.
Lebow et al62 provide animal and human support that PTSD susceptibility is linked to genetic and epigenetic changes in GR pathways. Focusing on glucocorticoid-induced leucine zipper (GILZ), a transcription encoded on the X chromosome by the Tsc22d3 gene, human and mouse data revealed very significant GILZ alterations in those exposed to a stressor in early life, adulthood, or both. In humans, the number of traumatic events negatively correlated with GILZ messenger RNA (mRNA) levels and positively with % methylation only in males. In mice, the number of stress exposures proportionally reduced GILZ GR pathway markers in the amygdala. GILZ “knockdown” (an experimental reduction in gene expression through genetic manipulation or the administration of a reagent) affected dendritic spine quality in the hippocampus and cortex that could explain PTSD-related changes in brain connectivity and hippocampal volume.
Very recent PTSD investigations are specifically targeting military samples. Wang et al63 conducted a genome-wide study of over 2000 Danish soldiers 6-months following deployment to Afghanistan, the Balkans, or Iraq. They found one region, 4q31—close to the Interleukin-15 gene (IL15 gene: a cytokine involved in the activation and proliferation of T-cell and “Natural killer cells” central to immune system function) was specifically associated with PTSD. They concluded that genetic perturbations of the inflammatory response may play a role not only in PTSD, but between it and correlated psychiatric disorders including depression, insomnia, and schizophrenia. Remaining questions include whether the association between inflammation and PTSD is specific, a more general reflection of mood and anxiety disorders, or if inflammation is a consequence of trauma exposure and not PTSD per se.
Genetic overlap between PTSD and other psychiatric and medical conditions was further suggested by the findings of another recent genome-wide study of over 165 000 U.S. military veterans.64 Eight separate genome regions were associated with PTSD re-experiencing, the disorder’s most distinct symptom cluster (see above). Several genome regions were particularly significant: gene CAMKV, a region near genes KANSL1 and CRHR1, and gene TCF4. CRHR1 is involved in the body’s steroid-hormone stress response. There was also genetic support for relationships between PTSD and hypertension (a common comorbid condition), as well as between re-experiencing symptoms and schizophrenia and bipolar disorder. Gelernter et al propose that the nightmares and flashbacks experienced by persons with PTSD may share a common biochemical pathway with hallucinations common to schizophrenia. While not specifically mentioned, it is important to note that serious PTSD in OEF/OIF/OND soldiers commonly includes paranoid symptoms, another characteristic common in certain forms of schizophrenia.
These few studies are but a small sample of recent research into the epigenesis of PTSD. Promising findings support genetic connections with HPA axis reactivity and other psychiatric and medical conditions—particularly those in which inflammation plays a role. As discussed above, one of the direct consequences of blast TBI is inflammation. Given this, one could propose a potential diathesis-stress relationship between blast and PTSD onset—particularly in those soldiers whom are genetically predisposed. We now begin a more direct comparison between blast-induced TBI and PTSD to further examine potential comorbid relationships.
Human Imaging Studies
It is been argued that blast wave head trauma and PTSD have a negative impact on the same brain structures.65 These regions include the hippocampal/amygdalar complex and those cortical structures which regulate it. Imaging studies of OEF/OIF/OND veterans have generally relied on fMRI or diffusion tensor imaging (DTI) methodologies. DTI measures the relationship between axial and radial water diffusion that reflects the organization and integrity of white matter (ie, myelinated axons).
Findings purported to reveal the intra-diagnostic effects of PTSD and TBI have varied substantially across studies.65-80 Furthermore, obvious inter-diagnostic differences are absent. Table 1 summarizes the selection of PTSD studies and their results; Table 2 presents the mTBI and comorbid PTSD/TBI studies. For PTSD studies, brain abnormalities were often proportional to symptom severity; in TBI studies abnormalities were more frequent in soldiers who had experienced LOC.
Table 1.
Summary of select imaging studies of veterans with PTSD.
| Authors | Methodology | Significant findings | Functional implications |
|---|---|---|---|
| Hayes et al66 | Structural MRI | Reduced CA4/dentate volume; inverse relationship with symptom severity. | Impaired declarative memory. |
| Rabinak et al67 | Resting state fMRI | Strong functional coupling between the insula & right amygdala. | Basal hyperarousal and hypervigilance; insula relays interoceptive information to guide fear responses. |
| Kennis et al68 | Resting state fMRI | Alterations in the salience, default mode and central executive networks. | Potential alteration in detecting and filtering salient stimuli; heightened introspection and interior states; diminished self-control, altered reappraisal of threats and increased intrusive/unpleasant thoughts. |
| Yuan et al70 | Resting state fMRI and EEG | Heightened default mode activation positively related to PTSD symptoms; lower activation of the salience network. | Exacerbation of collective PTSD symptoms arising from insufficient top-down limbic modulation. |
| Badura-Brack et al71 | Structure MRI and MAG; presentation of angry & neutral faces | Stronger and faster left-sided amygdala reactivity reflecting a bottom-up amygdala drive on cortical functioning. | Heighten behavioral response to perceived threats. |
| Sanjuan et al72 | DTI | Lower FA in bilateral dorsal cingulum and right anterior corona radiate (ACR). ACR inversely related to symptom severity. | Heightened re-experiencing, avoidance, and arousal. |
| Averill et al74 | DTI | FA for the left cingulum angular bundle positively correlated with symptom severity. Possible negative impact on default mode network. | Possible hindrance of episodic memory, decision-making and executive control. |
| Lindemer et al75 | T1-weighted scans | Negative relationship between symptom severity and cortical thickness in postcentral and middle temporal gyri. Thinness in bilateral superior frontal regions related to comorbid TBI. | Possible impairment in aspects of somatosensory function, facial recognition, word comprehension, self-awareness, and possibly humor. |
| Wrocklage et al76 | T1-weighted scans | Gray matter cortical thickness negatively associated with total PTSD symptoms primarily across the left PFC. | Heightened symptoms of dysphoric arousal, re-experiencing, emotional numbing and behavioral avoidance. |
Abbreviations: ACR, anterior corona radiate; DTI, diffusion tensor imaging; EEG, electroencephalogram; FA, fractional anisotropy; fMRI, functional magnetic resonance imaging; MRI, magnetic resonance imaging; PFC, prefrontal cortex; PTSD, posttraumatic stress disorder; TBI, traumatic brain injury.
Table 2.
Summary of select imaging studies of veterans with TBI or comorbid TBI/PTSD.
| Authors | Methodology | Significant findings | Functional implications |
|---|---|---|---|
| Fischer et al77 | fMRI; “Stop Signal Task” | Hyperactivation in bilateral inferior temporal, left superior temporal, caudate, and cerebellar regions in blast TBI Veterans. | Cognitive impulsivity yielding commission errors that undermine information processing accuracy. |
| Depue et al65 | fMRI; continuous performance task | Reduced left amygdala volume. | Increased commission errors reflecting impaired impulse control. |
| Hayes et al78 | DTI | Diffuse white matter abnormalities with TBI that included LOC; lower FA with higher blast load in the left retrolenticular internal capsule; diffuse white matter abnormalities. | Possible hindrance of the transmission of visual and auditory information between lower and higher brain regions. |
| Rangaprakash et al79 | Resting State fMRI | An “aberrant pre-frontal-subcortical-parietal network of information flow” with specific foci—middle frontal gyrus (MFG), the insula, and hippocampus. | Predictive of PTSD symptoms and PCS. MFG dysregulation contributes to PTSD hyperarousal and re-experiencing. Potential cognitive and/or emotional difficulties secondary to MFG dysfunction. |
| Miller et al80 | DTI | TBI with LOC yielded the most diffuse white matter abnormalities. | TBI was associated with physical PCS and PTSD was associated with emotional and cognitive PCS. |
Abbreviations: DTI, diffusion tensor imaging; FA, fractional anisotropy; LOC, loss of consciousness; MEG, magnetoencephalography; MFG, middle frontal gyrus; PCS, postconcussive symptoms; PTSD, posttraumatic stress disorder; TBI, traumatic brain injury.
The specific limitations of these imaging studies are that they often employed small samples, that biographic/demographic details or variables such as number of deployments or time since last deployment were not considered, and that the need to control for multiple analyses diminished statistical power. Perhaps the greatest weakness of the “PTSD-only” studies is that the authors believed that excluding veterans who had suffered moderate and severe head injury was adequate to control for TBI effects. As previously introduced, most head injuries to GWoT soldiers were mild and yet they commonly experience chronic PCS suggestive of postconcussive syndrome. There is also the strong possibility that mTBI plays a significant role in precipitating some cases of PTSD (see below “Blast-Induced PTSD”). Contrarily, most TBI studies only examined veterans with mild head injury.
Integrated summary across diagnoses and studies
Significant unilateral findings from clinical samples are predominately within the brain’s left hemisphere and include (1) stronger and faster amygdala reactivity and reduced amygdala volume and diffuse white matter abnormalities in the retrolenticular internal capsule (largely containing optic radiations involved in to-and-fro cortical communication). (2) Cortical thinning in the dorsal anterior cingulate cortex (the detection and appraisal of social processes), the cingulum angular bundle (decision-making and other executive functions), dorsolateral PFC (attentional networks that support cognitive selection of sensory information and response, and possibly emotional reactivity), and the anterior insula (processes the sense of disgust for smells and images, as well as societal norm violations). (3) Hyperactivation in the superior temporal (auditory and language processing; social cognition), caudate (the evaluation of past experiences to influence future behavior), and cerebellar (the learning and coordination of voluntary motor behaviors) regions. While less frequent, right-sided abnormalities include a strong functional coupling between the insula and the amygdala, and cortical thinning in the anterior corona radiate (promotes communication between the cortex and brain stem), lateral occipital cortex (object recognition), fusiform (facial and color recognition), and the posterior cingulate (awareness, pain, working memory, and episodic memory).
Bilateral differences between clinical and control samples were observed in (1) the ventrolateral (response inhibition and goal-appropriate responding) and superior PFC (self-awareness and humor); (2) across the orbitofrontal (response inhibition, impulse control, decision-making), temporoparietal (processing of multisensory information, self-other distinctions, moral decisions), and anterior cingulate cortex (autonomic functions, attention allocation, reward anticipation, decision-making, and morality); (3) between the default mode (DMN) and salience (SN) networks, the postcentral (primary somatosensory cortex) and middle temporal gyri (judging distance, facial recognition and reading), and superior frontal regions. As introduced above, the SN is involved in detecting the most goal-relevant information from competing stimuli. The DMN is activated when the individual is thinking about him/herself and his or her relationship to the past, present, and the future and is based in the ventromedical PFC and the posterior cingulate cortex.69
Regarding the interpretation of imaging findings and in line with the CWS model, it has been proposed that TBI contributes to a “neural environment in which the brain is more susceptible to stress induced damage, or alternatively, damages brain regions that result in greater expression of PTSD symptoms.”75 This is supported by a significant negative relationship between the severity of combat exposure and left lateral PFC thickness in veterans without PTSD.76 This finding along with those from veterans with PTSD suggest that combat in and of itself has lasting effects on the brain—likely mediated by HPA axis overactivation and the negative impact of factors such as protracted sleep deprivation.6 We argue that such findings strongly support the CWS model which maintains that the stressors of war have a negative impact on the brain creating CNS vulnerability that interacts with the individual’s strengths, weaknesses, and unique experiences which are then compounded by deployment cycles and post-deployment environments.
Diagnostic and Symptomatic Relationships
TBI and PTSD
Supported by imaging study findings, there is substantial overlap between TBI and PTSD symptomatology that can make diagnostics difficult. Both conditions commonly include insomnia, fatigue, irritability, depression, anxiety, emotional numbing, avoidance, trouble concentrating, memory deficits, derealization (ie, a sensation/perception that one’s surroundings are not real), depersonalization (ie, feeling detached from one’s mind and/or body), and hyperarousal.81 While they can occur in both conditions, headache, dizziness, and light and sound sensitivity are more common following TBI. Re-experiencing and feelings of shame and guilt occur more frequently in PTSD.
As many as one-half of OEF/OIF/OND soldiers with combat-related mTBI meet PTSD diagnostic criteria;28 furthermore, injury and particularly TBI appears to predict PTSD. Hoge and colleagues found that 44% of 2525 OIF soldiers who experienced blast-related LOC met PTSD criteria, 27% with blast-related AOC met criteria, 16% who experienced other injuries met criteria, and only 9% of soldiers with no injury met PTSD criteria.82 Furthermore, veterans who sustained multiple head injuries had significantly higher rates of PTSD, depression, and suicidal ideation.28 A 2.37 prevalence ratio has also been established between combat-related mTBI and PTSD.83 A study of over 27 000 Special Operation soldiers found those who suffered a combination head injury (ie, blast and blunt force) evidenced the highest level of PTSD symptoms, followed by those with just a blunt force injury, and those with no injury.84
What is not obvious is whether the association between head trauma and PTSD reflects a physical or psychological pairing.7 The blast explosions experienced by GWoT soldiers impact the entire brain; therefore, the possibility of developing cognitive, emotional, and ANS symptomatology might be greater in instances of more significant head injury as evidenced by LOC. Contrarily, since the head is usually associated with the seat of consciousness and the self, a head injury—especially one that includes AOC or LOC, might be viewed as a more life-threatening event and thus more likely to evoke PTSD symptomatology. Recent animal and human research provided support that in some instances TBI can precipitate PTSD. Those findings are introduced below.
Blast-induced PTSD
Kennedy et al85 also concluded that PTSD in OEF/OIF/OND soldiers is strongly related to head trauma. Approximately 40% of active duty soldiers with blast and non-blast head injuries reported clinically significant PTSD systems; however, the blast injury group reported significantly more re-experiencing symptoms. As discussed above, the re-experiencing cluster contains the most diagnostically distinct PTSD symptoms.
It has been argued that one could not develop PTSD after a head injury that included AOC/LOC/PTA since it would preclude memory formation.86 The current view is there are several pathways to PTSD following TBI: unconscious encoding of the emotional and sensory qualities of the traumatic experience, conscious encoding of some aspects of the event, reconstructing memory of the head injury from the report of others, and developing PTSD in reaction to ongoing and related events even if there is no memory for the specific details of the head injury.
Shared neural substrates
McAllister and Stein87 reviewed the neural substrates common to TBI and PTSD. Mesial temporal structures (ie, amygdala, hippocampus, uncus, dentate gyrus, and parahippocampal gyrus) are vulnerable to TBI from contact and impact forces and increased sensitivity to excitotoxicity (ie, neuronal injury or death from neurotransmitter overstimulation). The orbitofrontal cortex which plays an important role in response inhibition, impulse control, emotion, reward, and decision-making is also vulnerable from direct impact or damage to frontal-subcortical projections (eg, the medial dorsal nucleus of the thalamus). McAllister and Stein87 specifically proposed that biomechanical and neurochemical insult could interact with “neurohumoral dysregulation” (ie, HPA axis over-reactivity) to create a milieu that promotes the development of PTSD. From the perspective of the CWS model, such interactions would be bidirectional5: The chronic stress of deployment promotes CNS vulnerability (especially within the limbic-cortical circuitry that underlies PTSD and plays a role in fear conditioning and contextual memory consolidation [see above]) so that the impact of even minor TBI is exacerbated, and the consequences of the head injury would further promote HPA over-reactivity. From their rodent research Perez-Garcia et al88 offered a similar hypothesis: Blast injury may precipitate PTSD traits carried by the individual or the blast directly injures brain structures that are involved in coping with significant psychological stress, thus predisposing the soldier to develop PTSD.
Supportive rodent research
In a very intriguing rodent study, Elder and colleagues examined the effect of repetitive blast injury on anesthetized rats and found that it precipitated a variety of PTSD-related behaviors including anxiety, enhanced contextual fear conditioning, and an altered response in a predator scent assay; furthermore, these behaviors persisted for months and were associated with protein stathmin 1 (involved in the regulation of the microtubule filament system [maintenance of cell structure]) elevations in the amygdala.89 The researchers concluded that since the rats were unconscious when subjected to overpressure blasts, PTSD-like behaviors can develop without a psychological stressor.
Perez-Garcia and colleagues recently published two important studies that further support a relationship between blast injury and PTSD, point to the underlying biochemistry, and even recommend a novel pharmacological therapy. The researchers proposed that most rodent studies examining blast injuries focus on acute to subacute exposures that are too intense—being comparable to moderate-to-severe head injuries in humans.90 Utilizing a similar methodology as Elder et al,89 anesthetized rats were exposed to repetitive low-level overpressure attempting to model the experiences of deployed OEF/OIF/OND soldiers. The rats demonstrated a variety of anxiety and “PTSD-related” behavioral traits. Of significance is that these rats continued to exhibit exaggerated fear responses between 28 and 35 weeks after the final blast exposure. The authors concluded that their findings offer a rat model of how repetitive low-level blasts can induce chronic PTSD in the absence of psychological stressors and may further our understanding of the etiology of comorbid postconcussive syndrome and PTSD.
Again, using their mTBI protocol, Perez-Garcia et al91 administered “low” and “high” doses of BCI-838 to some of the rats exposed to a series of low-level blasts. BCI-838 is a Group II metabotropic glutamate receptor antagonist that is currently being studied in humans for refractory depression and suicidality. Its active metabolite BCI-632 has proneurogenic, procognitive, and antidepressant effects in animals and has been found to diminish anxiety and improve memory in an animal model of Alzheimer’s disease. BCI-838 was shown to reverse PTSD behavioral traits, diminish anxiety and fear-related behaviors, and improved long-term recognition memory in the blast-exposed rats. The low dose group showed improvement over the blast-only group and those rats in the high dose group demonstrated behavior comparable to the non-blast controls. Furthermore, the brains of sacrificed rats treated with BCI-838 showed increased neurogenesis in the hippocampus, particularly in the area of the dentate gyrus (DG). The DG contributes to learning, memory, and spatial coding. Perez-Garcia et al91 propose that since BCI-838 can simultaneously enhance DG neurogenesis and diminish multiple PTSD traits, glutamatergic components such as those in the hippocampus and the cortex play an important etiological role. They further suggest that BCI-838 could be a promising drug for soldiers and veterans suffering from PTSD who have an mTBI history.
These investigations support the value of animal models for understanding the complexities of blast wave injury on the brain. The findings that mTBI can precipitate PTSD-like behaviors in rats in the absence of psychological stressors appears to support the established relationship between head injury severity and PTSD in OEF/OIF/OND soldiers and that those soldiers who suffered LOC are more often diagnosed with the disorder. In a solid review of the human and animal literature, Elder et al92 propose that GWoT service members who have been diagnosed with comorbid postconcussive syndrome and PTSD may actually suffer from a single disorder on the “spectrum of blast-related brain injury.”
As previously introduced, a potential mediator between blast-related head injury and PTSD may be deployment-related HPA axis over-reactivity and its ensuring inflammation and autoimmune response. This response would be compounded by the occurrence of a TBI and its own but similar aftereffects. This neurohumoral cascade would then drive additional changes in genetic transcriptions and the alteration of the neural substrates common to both disorders. Furthermore, it is possible that the eventual severity of PTSD symptoms would be proportional to the intensity of the blast and whether it produced an AOC or LOC. While entirely speculative, there may be an interaction between a soldier’s genetic predisposition toward PTSD and the blast’s impact on consciousness so that soldiers who are more prone require a less intense explosion to develop the disorder.
TBI, PTSD, and PCS
We return our attention to human symptomatology to examine the apparent contributions of head injury and/or PTSD to PCS and neuropsychological deficits. The investigations presented in this and the following section usually relied upon self-reported historical events (eg, head injury specifics), psychometric evaluation of current symptomatology (eg, PCS, PTSD, depression) and cognitive functioning (eg, tests of attention, memory, processing speed), and used statistical modeling to determine the specific contributions of TBI and PTSD.
As previously introduced, PCS are very common in OEF/OIF/OND soldiers experiencing the consequences of war. That there is considerable overlap with PTSD symptoms is not surprising given their established relationship to military head injury. What has been in question is whether PCS are caused by TBI, psychiatric factors or both.81
Baldassarre et al2 reported findings supportive of PCS arising from mTBI. OEF/OIF veterans with a history of head injury acknowledged more vestibular (59%), somatic (34%), cognitive (22%), and affective symptoms (15%) than soldiers without mTBI. Further support was reported by Williams et al93 who found that PTSD did not mediate relations between PCS and cumulative disease burden and that PCS have a direct impact on veterans’ health above and beyond PTSD. Despite the findings of these two studies, most recent investigations support PTSD symptoms playing an important role in PCS.
Andrews et al94 found that psychiatric and behavioral conditions including PTSD independently accounted for 42.5% of the variance in PCS compared to 1.5% for TBI. Another study found no significant PCS differences in OEF/OIF/OND veterans with and without a TBI history.95 Furthermore, regression analysis revealed that symptoms of PTSD and depression were the strongest predictors of PCS even when the overlapping symptoms of the two disorders were removed. Similarly, it was found that PTSD served as a mediator between PCS and pain severity and its functional impact in GWoT veterans.96 Pietrzak et al97 also reported that PTSD mediated the relationship between mTBI and all the study’s outcome measures including overall health, unmet medical and psychological needs, measures of psychosocial difficulties, and perceived barriers to behavioral health care. Lippa et al98 examined out-patient veterans who reported blast-related mTBIs, non-blast mTBIs and those who had both. Across patients there was no difference in PCS severity or symptom profile. In blast-related patients, PCS did not vary significantly by the number of mTBIs they reported or by their proximity to the explosions; instead, PTSD accounted for a substantial portion of PCS variance. Exploratory linear regression of 139 of our own patients found that PTSD symptom-severity accounted for 52% of the variance while the total number of self-reported TBI AOC/LOC experiences accounted for only 12%. Lippa et al98 concluded that the primary clinical focus should be upon those factors that are currently responsible for patients’ symptoms rather than emphasizing the contribution of a remote TBI. We have also found this approach most successful for our CWS patients6 (see below).
The findings presented in the last three sections suggest the following conclusions: There is strong support for a relationship between TBI and PTSD in GWoT soldiers. In some cases, TBI may directly precipitate PTSD symptoms. While not intuitive, the relationship between TBI and PCS appears indirect and mediated through PTSD. We now turn to the effects of blast exposure and PTSD on the cognitive functioning of soldiers and veterans.
Neuropsychological Functioning
Overview
Neuropsychology is the study of brain–behavior relationships and the application of that knowledge to clinical and research endeavors. Psychometric tests, presented directly to the examinee or via computer, assess a variety of cognitive domains (eg, attention and mental flexibility, processing speed, memory, sensory and motor functioning and higher-order reasoning). Numerous comingling psychological and physical factors should be considered when interpreting neuropsychological test results—especially in soldiers/veterans experiencing the consequences of war. Head injury, PTSD, chronic pain, insomnia, depression, and anxiety can singularly affect cognitive functioning and collectively, their impact is likely additive if not exponential.6
Most of the studies reviewed did consider the psychiatric status of participants even though the primary goal was to examine mTBI effects. Usually this entailed statistical control, or specific regression analysis of PTSD effects. Several studies formed separate groups to examine the singular or joint effects of mTBI and PTSD.
Most of the investigations failed to find pervasive or serious neuropsychological impairment in OEF/OIF/OND soldiers/veterans; identified deficits were usually related to information processing and executive functions. Of particular significance is that the consensus of studies concluded that head injury history did not contribute substantially to current cognitive deficits.
We also have found active duty service members’ difficulties are predominately with information processing and EFs rather than serious deficits within or across major functional domains.5,6 Computerized tests are particularly sensitive for documenting such difficulties because they challenge the individual’s ability to self-regulate the quality and accuracy of information processing. We consistently observe a response pattern where soldiers show slowed information processing but are often impulsive in their responding and do not take the time needed to ensure encoding. The two factors interact with slowed processing reducing the efficiency of information processing and impulsivity hindering accuracy. Furthermore, the degree of these problems is strongly associated with the range and severity of patients’ current self-reported physical and emotional symptoms.
Remote TBI versus PTSD
A sample of the studies reviewed found: mTBI was not responsible for poor performance on measures of attention, information processing, working memory, and mental calculation; instead, cognitive deficits were significantly related to current PTSD symptom-severity;99,100 no significant difference was observed between veterans in a mTBI-only condition and the control group;100 after controlling for their PTSD symptomatology, group differences disappeared between veterans with past TBI and control participants,101,102 and that factors such as number of lifetime concussions, and if the veteran experienced AOC versus LOC did not affect cognitive test performance.103 The conclusions offered by a number of the authors could be quite strong and included: That neurocognitive differences in OEF/OIF veterans might be better explained by PTSD than blast exposure history; in agreement with other investigations remote combat-related mTBI does not in and of itself contribute to objective cognitive impairment,104 and findings fall within those of numerous meta-analyses conducted on general TBI data sets that acute neurocognitive effects resolve within several weeks to months and that there is no dose-response relationship between the effects of a single and multiple concussions on neuropsychological functioning.105
Clinical Implications and Application of the CWS Model
Overview
Many active duty OEF/OIF/OND soldiers remain seriously hindered by the constitute chronic conditions that define the CWS including physical pain and insomnia, other somatic conditions such as visual and vestibular dysfunction, cognitive deficits, and some degree of PTSD symptomatology, depression, and anxiety. Furthermore, these disorders can continue to plague service members and impact their families long after they leave the military.
As evident throughout this review, we maintain there is an etiological and clinical symbiosis between the CWS diagnoses so that their collective whole is more pernicious than if the soldier or veteran has only one or even a subset. What we have found particularly remarkable is how similar the clinical presentation is across the several thousand soldiers for whom we have provided care at a number of military treatment facilities (MTF) in and outside the continental United States over the last decade. Despite varying in age, gender, education, rank, MOS (occupation), number of combat deployments, and even head injury history, it is not whether the service members are experiencing the entire constellation of CWS disorders, it is to what degree they are experienced.
It is important to note that we have always served at specialty clinics generally designated for TBI. Thus, there is some selection bias whereby those soldiers referred to us have generally suffered from the constitute CWS conditions for some time and may have exhausted less comprehensive approaches to care. For instance, of the last 139 patients who have completed our 6-week intensive outpatient program (IOP),6 it had been nearly 4 years since their last deployment and when they began IOP care they acknowledged severe-to-very severe headache impact, moderately severe PTSD symptoms and depression, moderate-to-severe anxiety, moderate insomnia, somatic and vestibular symptoms, and moderate functional cognitive deficits. Their presenting level of distress and its persistence across time conforms to the tenants of the CWS model which posit complex interactions between the inherent stresses of deployment, difficulties reintegrating into post-deployment life, the onset of the comorbid disorders, and their exacerbation and growing chronicity over time.
Philosophy of care and patient education
The CWS model is essential to our clinical philosophy, drives our program of care, and is shared by a clinical staff of over 30 first- and second-tier providers. The suffering of soldiers can be significantly ameliorated through a transdisciplinary course of care that addresses each of the major CWS disorders. Under the guidance of solid case management, care is coordinated across a variety of disciplines consisting of neurology; medical management; pain management; rehabilitation services, including neuro-optometry, occupational, physical, and speech therapy; and behavioral health—be it through psychotropic medication and/or a variety of individual/group therapies. Improvement can come through individual provider appointments and/or from an IOP lasting several weeks.6 Because of the complex symbiosis across the physical, emotional, and cognitive conditions, achieving some success with each disorder contributes to the improvement of the other disorders and to the global recovery of the soldier (or veteran). Furthermore, a rigorous assessment program can empirically substantiate transdisciplinary care and establish evidence-based interventions.5-7
As patients move through care, their understanding of CWS is established and fortified through provider encounters: (1) They are introduced to the idea that their difficulties commonly began during deployment (s) with particular emphasis on the effects of chronic stress and the onset and/or exacerbation of chronic insomnia and pain, as well as the impact of specific physical and emotional trauma. (2) The potential vulnerabilities initiated through deployment (s) are discussed, particularly with regard to how they interact with the significant change in social, emotional, cognitive and even occupational demands that comes with post-deployment reintegration. (3) The transition from more acute to chronic symptomatology is explained, as well as accounting for which disorders are responsible for the soldier’s various functional difficulties. Finally, (4) the transdisciplinary model of care is introduced, treatment options are discussed, and expectations offered—often supported by actual empirical findings obtained from our program evaluation efforts. Of course, the approach to education is tempered by where the soldier is in his or her course of treatment, present level of functioning, and in consideration of their personal strengths and weaknesses.
As will be discussed below, an important component of our transdisciplinary care is offering patients a reconceptualization of their deployment and post-deployment experiences and the role which they played then and now. While we certainly acknowledge the important contributions of the patient’s history in the etiology and maintenance of CWS, we predominately focus upon his or her current symptomatology and present therapeutic efforts. One important reason is that it is quite common for service members to place an incorrect emphasis on the remote and preponderantly mild TBIs they experienced during deployment as being the primary source for their chronic and often worsening difficulties. This is understandable since blast exposures and/or impact head injuries are events that particularly standout in time. We consistently observe that reframing the role of mTBI in CWS diminishes the pessimism often held by soldiers that their chronic conditions are directly tied to serious brain damage from which they will “never recover.” By emphasizing the ongoing contributions of experiential and environmental factors to their difficulties offers tempered hope that they can be in a much better place than when they began transdisciplinary care.
The “war-within”
Theoretical perspective
To this point, we have primarily described the biological mechanisms contributing to the phenomena of CWS. We now direct our attention to the phenomenology of those whom suffer the consequences of war.
The psychological costs of deployment and its aftermath can be profound. Many of our patients continue to fight a “war-within” (W-WI).7 Metaphorically, they carry on a psychological battle with an enemy who has taken up residence within them. Consequentially, the homeland is now experienced as dangerous, chaotic, and intrusive. Soldiers’ constant vigilance and situational awareness rivals that of what was required for survival when deployed. Further difficulties arise from interactions with a civilian population that lacks a frame of reference and cannot understand the soldier’s experience of war. Service members then commonly separate themselves from loved ones, friends, and comrades, come to see a world where they no longer fit in, and even contemplate if life is worth living. The essence of the W-WI concept lies in the discrepancy between the soldier’s “Pre-Deployment” and “Post-Combat Warrior Self-Narrative” driven by his or her actual experiences of war versus pre-deployment expectations and training.
An integration of the clinical insights offered by Narrative Psychology and Constructivist Self-Development Theory has been particularly useful for developing the W-WI model. The two theories have contributed to our problem formation, case conceptualization, and approach toward clinical intervention.
Narrative Psychology proposes that people build internal stories to find, create, and make meaning of their experience.106 The construction of a personal narrative can be both positive and negative, can change or adjust over time, and is informed by the culture and the environment the individual inhabits. One’s identity and sense of self is made and remade by the stories the individual, others, and the culture tell about us. Therefore, trauma and the person-environment interaction can challenge the adequacy of the survivor’s self-narrative and lead to a revision that more accurately reflects how trauma has changed their life. From this perspective, clinicians can potentially uncover the psychological consequences of war by juxtaposing the service member’s Pre-Deployment and Post-Combat Warrior Self-Narrative. Clinical interventions center on externalizing the problem, assessing what the problem has taken away from the individual, and determining how the individual can gain mastery.
Constructivist Self-Development Theory integrates object relations, self-psychology, and social cognition theories to help understand the person-environment interaction.107 Specific emphasis is placed on the person being an active creator of their reality. As the individual interacts with the environment they receive and interpret feedback which informs their understanding of the self, others, and the world. McCann and Pearlman highlight how trauma can disrupt the survivor’s self-capacities (ie, ability to tolerate strong affect and regulate self-esteem), ego resources (ie, awareness and perspective taking), cognitive schemas (ie, beliefs and expectations about the self and others), and psychological needs (ie, safety, trust, esteem, intimacy, power, and independence).
Clinical conceptualization
For some returning soldiers, war and its traumatic nature can alter the individual’s basic assumptions about self, others, and the world. Prior to deployment they may have operated under the Pre-Deployment Warrior Self-Narrative whereby they experienced themselves as competent, strong, fearless, and able to exert their will. After deployment, the Post-Combat Warrior Self-Narrative may engender perceptions of impotency, weakness, failure, and incompetence. Prior to deployment, “Others” may have been seen as loving, dependable, and trustworthy. After deployment, Others may be experienced as indifferent, unreliable, and disingenuous. Before war, the “world” was safe, predictable, and manageable; upon returning home, it is transformed into a dangerous and unpredictable reality to be avoided. Metaphorically, we would argue that for some soldier’s the Post-Combat Warrior Self-Narrative has been co-opted and weaponized by the enemy within and needs to be explicitly and aggressively targeted in treatment.
Intervention
In order to win the war-within, a fundamental shift is required in how the Post-Combat Warrior Self-Narrative is understood coupled with a reconceptualization of the consequences of war. Providers would benefit from embracing the military culture and tailoring interventions with language and metaphors that match the every-day lives of the soldier.
An important component in therapy is the notion of the “Warrior Ethos” which the Army defines through the following core statements: “I will always place the mission first; I will never accept defeat; I will never quit; I will never leave a fallen comrade.” The Warrior Ethos is the crystalized representation of what is expected of the service member and the associated language that informs how he or she is to be evaluated and measured. The professional soldier seeks to not only embody the warrior narrative and its associated metaphorical representations, but to literally actualize its aspirations through acts of courage, honor, strength, loyalty, and selfless service.
Our patients consistently report that the W-WI model and the associated military-centric language helps them better understand the “why” at the root of their difficulties. By reframing the issues as something metaphorically created by the enemy and not a self-condemnation of their being “defective,” “broken,” or “inadequate” empowers them to fight. The Warrior Ethos becomes reinvigorated and the competitive nature of the soldier can be awoken. Service members are challenged to objectively look at the Warrior Ethos and its associated demands and expectations, understand the importance and relevance of the ethos in theater, critically examine the context surrounding seminal events from theater, and identify the vulnerability of the ethos after war that can create false expectations metaphorically exploited by the enemy within. By transplanting their internal problems and issues outside of themselves we are able to focus on what the enemy has metaphorically done to soldiers, has taken from them, and what they and the care team can accomplish together to help soldiers learn the coping skills necessary to win the war-within.
Finally, our patients are asked to identify and specifically define what wining looks like and are taught strategies to help achieve this end. Ultimately, treatment represents the training and arming of warriors with the psychological weapons required to win the war-within. The clinical strategies and techniques learned in training/treatment are housed in the service member’s armory of weapons and can be strategically deployed as needed to ensure their victory over the consequences of war.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Both authors contributed to theoretical formulations presented in this article. Dr. Dieter contributed more to the idea of a Consequence of War Syndrome (CWS) and Dr. Engel to that of a War-Within (W-WI). Dr. Dieter was the primary author of sections related to CWS and Dr. Engel to W-WI. Both authors edited the manuscript.
Disclaimer: The views expressed in this publication do not reflect those of the Department of Defense and are solely those of the authors.
ORCID iD: John NI Dieter  https://orcid.org/0000-0003-3780-390X
https://orcid.org/0000-0003-3780-390X
References
- 1. DePalma RG, Hoffman SW. Combat blast related traumatic brain injury (TBI): decade of recognition; promise of progress. Behav Brain Res. 2018;340:102-105. [DOI] [PubMed] [Google Scholar]
- 2. Baldassarre M, Smith B, Harp J, et al. Exploring the relationship between mild traumatic brain injury exposure and the presence and severity of postconcussive symptoms among veterans deployed to Iraq and Afghanistan. PM R. 2015;7:845-858. [DOI] [PubMed] [Google Scholar]
- 3. Morissette SB, Woodward M, Kimbrel NA, et al. Deployment-related TBI, persistent postconcussive symptoms, PTSD, and depression in OEF/OIF veterans. Rehabil Psychol. 2011;56:340-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mortera MH, Kinirons SA, Simantov J, Klingbeil H. Long-term neurobehavioral symptoms and return to productivity in Operation Enduring Freedom/Operation Iraqi Freedom veterans with and without traumatic brain injury. Arch Phys Med Rehabil. 2018;99:S50-S57. [DOI] [PubMed] [Google Scholar]
- 5. Dieter JNI, Engel SD. The efficacy of a transdisciplinary intensive outpatient program for treating active duty service members with TBI and associated disorders. In: FY17 Prevention, Mitigation, and Treatment of Blast Injuries Report to the Executive Agent. Fort Detrick, MD: US Department of Defense Blast Injury Research Program Coordinating Office; 2018:194-196. [Google Scholar]
- 6. Dieter JNI, Engel SD, Yarvis J. The Intrepid Spirit Center. In: Weiss EL, Castro CA, eds. American Military Life in the 21st Century: Social, Cultural, Economic Issues and Trends. Santa Barbara, CA: ABC-CLIO; 2018:282-295. [Google Scholar]
- 7. Dieter JNI, Engel SD, Yarvis J, Sharrieff AM. PTSD in service members and veterans. In: Weiss EL, Castro CA, eds. American Military Life in the 21st Century: Social, Cultural, Economic Issues and Trends. Santa Barbara, CA: ABC-CLIO; 2018:205-218. [Google Scholar]
- 8. Morena M, Patel S, Bains JS, Hill MN. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology. 2016;41:80-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Bodegom M, Homberg JR, Henckens MJAG. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci. 2017;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. The impact of stress on body function: a review. EXCLI J. 2017;16:1057-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McEwen BS. Neurobiological and systemic effects of chronic stress [published online ahead of print April 10, 2017]. Chronic Stress (Thousand Oaks). doi: 10.1177/2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kinlein SA, Wilson CD, Karatsoreos IN. Dysregulated hypothalamic-pituitary-adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Front Psychiatry. 2015;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. 2014;94:1816-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Germain A, Richardson R, Stocker R, et al. Treatment for insomnia in combat-exposed OEF/OIF/OND military veterans: preliminary randomized controlled trial. Behav Res Ther. 2014;61:78-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plumb TR, Peachey JT, Zelman DC. Sleep disturbance is common among servicemembers and veterans of Operations Enduring Freedom and Iraqi Freedom. Psychol Serv. 2014;11:209-219. [DOI] [PubMed] [Google Scholar]
- 16. Institute of Medicine (US) Committee on Sleep Medicine and Research. Extent and health consequences of chronic sleep loss and sleep disorders. In: Colten HR, Altevogt BM. eds. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: National Academies Press; 2006. https://www.ncbi.nlm.nih.gov/books/NBK19961/ [PubMed] [Google Scholar]
- 17. McAndrew LM, D’Andrea E, Lu SE, et al. What pre-deployment and early post-deployment factors predict health function after combat deployment?: a prospective longitudinal study of Operation Enduring Freedom (OEF)/Operation Iraqi Freedom (OIF) soldiers. Health Qual Life Outcomes. 2013:11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li G, Zhang X, Zhang J, Wang E, Zhang H, Li Y. Magnetic resonance study on the brain structure and resting-state brain functional connectivity in primary insomnia patients. Medicine (Baltimore). 2018;97:e11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santarnecchi E, Del Bianco C, Sicilia I, et al. Age of insomnia onset correlates with a reversal of default mode network and supplementary motor cortex connectivity. Neural Plast. 2018;2018:3678534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Minkel J, Moreta M, Muto J, et al. Sleep deprivation potentiates HPA axis stress reactivity in healthy adults. Health Psychol. 2014;33:1430-1434. [DOI] [PubMed] [Google Scholar]
- 21. Vargas I, Vgontzas AN, Abelson JL, Faghih RT, Morales KH, Perlis ML. Altered ultradian cortisol rhythmicity as a potential neurobiologic substrate for chronic insomnia. Sleep Med Rev. 2018;41:234-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. Chronic insomnia and stress system. Sleep Med Clin. 2007;2:279-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGarity S, Brenner LA. Traumatic brain injury. In: Weiss EL, Castro CA, eds American Military Life in the 21st Century: Social, Cultural, Economic Issues and Trends. Santa Barbara, CA: ABC-CLIO; 2018:231-243. [Google Scholar]
- 25. Prince C, Bruhns ME. Evaluation and treatment of mild traumatic brain injury: the role of neuropsychology. Brain Sci. 2017;7:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DePalma RG. Combat TBI: history, epidemiology, and injury modes. In: Kobeissy FH, ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press/Taylor & Francis; 2015. https://www.ncbi.nlm.nih.gov/books/NBK299230/#ch2_sec1. [PubMed] [Google Scholar]
- 27. Haran FJ, Alphonso AL, Creason A, et al. Analysis of post-deployment cognitive performance and symptom recovery in U.S. PLoS ONE. 2013;8:e79595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lindquist LK, Love HC, Elbogen EB. Traumatic brain injury in Iraq and Afghanistan veterans: new results from a national random sample study. J Neuropsychiatry Clin Neurosci. 2017;29:254-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chapman JC, Diaz-Arrastia R. Military traumatic brain injury: a review. Alzheimers Dement. 2014;10:S97-S104. [DOI] [PubMed] [Google Scholar]
- 30. Tsao JW, Stentz LA, Rouhanian M, et al. Effect of concussion and blast exposure on symptoms after military deployment. Neurology. 2017;89:2010-2016. [DOI] [PubMed] [Google Scholar]
- 31. Mac Donald CL, Johnson AM, Wierzechowski L, et al. Outcome trends after US military concussive traumatic brain injury. J Neurotrauma. 2017;34:2206-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mac Donald CL, Barber J, Jordan M, et al. Early clinical predictors of 5-year outcome after concussive blast traumatic brain injury. JAMA Neurol. 2017;74:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nelson NW, Davenport ND, Sponheim SR, Anderson CR. Blast-related mild traumatic brain injury: neuropsychological evaluation and findings In: Kobeissy FH, ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press/Taylor & Francis; 2015. https://www.ncbi.nlm.nih.gov/books/NBK299235/. [PubMed] [Google Scholar]
- 34. Chen Y, Huang W, Constantini S. Concepts and strategies for clinical management of blast-induced traumatic brain injury and posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci. 2013;25:103-110. [DOI] [PubMed] [Google Scholar]
- 35. Cernak I. Blast injuries and blast-induced neurotrauma: overview of pathophysiology and experimental knowledge models and findings. In: Kobeissy FH. ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press/Taylor & Francis; 2015. https://www.ncbi.nlm.nih.gov/books/NBK299193/. [PubMed] [Google Scholar]
- 36. Przekwas A, Somayaji MR, Gupta RK. Synaptic mechanisms of blast-induced brain injury. Front Neurol. 2016;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heinzelmann M, Reddy SY, French LM, et al. Military personnel with chronic symptoms following blast traumatic brain injury have differential expression of neuronal recovery and epidermal growth factor receptor genes. Front Neurol. 2014;5:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 39. Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, Rosen RC. Posttraumatic stress disorder in veterans and military personnel: epidemiology, screening, and case recognition. Psychol Serv. 2012;9:361-382. [DOI] [PubMed] [Google Scholar]
- 40. Vincenzes KA. Comparison of civilian trauma and combat trauma. Vistas Online; 2013. https://www.counseling.org/knowledge-center/vistas/by-subject2/vistas-veterans/docs/default-source/vistas/comparison-of-civilian-trauma-and-combat-trauma.
- 41. Foy DW, Sipprelle RC, Rueger DB, Carroll EM. Etiology of posttraumatic stress disorder in Vietnam veterans: analysis of preliminary, military, and combat exposure influences. J Consult Clin Psychol. 1984;52:79-87. [DOI] [PubMed] [Google Scholar]
- 42. Taft CT, Schumm JA, Panuzio J, Proctor SP. An examination of family adjustment among Operation Desert Storm veterans. J Consult Clin Psychol. 2008;76:648-656. [DOI] [PubMed] [Google Scholar]
- 43. King PR, Donnelly KT, Warner G, Wade M, Pigeon WR. The natural history of sleep disturbance among OEF/OIF veterans with TBI and PTSD and the role of proxy variables in its measurement. J Psychosom Res. 2017;96:60-66. [DOI] [PubMed] [Google Scholar]
- 44. Wallace DM, Shafazand S, Ramos AR, et al. Insomnia characteristics and clinical correlates in Operation Enduring Freedom/Operation Iraqi Freedom veterans with post-traumatic stress disorder and mild traumatic brain injury: an exploratory study. Sleep Med. 2011;12:850-859. [DOI] [PubMed] [Google Scholar]
- 45. Mohlenhoff BS, O’Donovan A, Weiner MW, Neylan TC. Dementia risk in posttraumatic stress disorder: the relevance of sleep-related abnormalities in brain structure, amyloid, and inflammation. Curr Psychiatry Rep. 2017;19:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93:681-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fortier-Brochu E, Morin CM. Cognitive impairment in individuals with insomnia: clinical significance and correlates. Sleep. 2014;37:1787-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Colvonen PJ, Masino T, Drummond SP, Myers US, Angkaw AC, Norman SB. Obstructive sleep apnea and posttraumatic stress disorder among OEF/OIF/OND veterans. J Clin Sleep Med. 2015;11:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haba-Rubio J, Marti-Soler H, Tobback N, et al. Sleep characteristics and cognitive impairment in the general population: the HypnoLaus study. Neurology. 2017;88:463-469. [DOI] [PubMed] [Google Scholar]
- 50. Friedman MJ. PTSD history and overview. https://www.ptsd.va.gov/professional/treat/essentials/history_ptsd.asp. Accessed 2016.
- 51. Kennedy C, Wang S. Traumatic brain injury and post-traumatic stress disorder in military veterans: when two problems collide. National Center for Health Research. http://www.center4research.org/traumatic-brain-injury-post-traumatic-stress-disorder-military-veterans-two-problems-collide/. Accessed 2019.
- 52. Litz BT, Schlenger WE. PTSD in service members and new veterans of the Iraq and Afghanistan wars: a bibliography and critique. PTSD Res Q. 2009;20:1-8. [Google Scholar]
- 53. Schnurr PP, Lunney CA, Bovin MJ, Marx BP. Posttraumatic stress disorder and quality of life: extension of findings to veterans of the wars in Iraq and Afghanistan. Clin Psychol Rev. 2009;29:727-735. [DOI] [PubMed] [Google Scholar]
- 54. Shin LM, Rauch SL, Pitman RK. Structural and functional anatomy of PTSD findings neuroimaging research. In: Vasterling JJ, Brewin CR, eds. Neuropsychology of PTSD Biological, and Clinical Perspectives. New York, NY: Guilford Press; 2005:59-82. [Google Scholar]
- 55. Zotev V, Phillips R, Misaki M, et al. Real-time fMRI neurofeedback training of the amygdala activity with simultaneous EEG in veterans with combat-related PTSD. Neuroimage Clin. 2018;19:106-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J Clin Neurophysiol. 2017;34:300-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. To WT, De Ridder D, Menovsky T, Hart J, Vanneste S. The role of the dorsal anterior cingulate cortex (dACC) in a cognitive and emotional counting Stroop task: two cases. Restor Neurol Neurosci. 2017;35:333-345. [DOI] [PubMed] [Google Scholar]
- 58. Szczepanski SM, Knight RT. Insights into human behavior from lesions to the prefrontal cortex. Neuron. 2017;83:1002-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fama R, Sullivan EV. Thalamic structures and associated cognitive functions: relations with age and aging. Neurosci Biobehav Rev. 2015;54:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Benarroch EE. Locus coeruleus. Cell Tissue Res. 2018;373:221-232. [DOI] [PubMed] [Google Scholar]
- 61. Blacker CJ, Frye MA, Morava E, Kozicz T, Veldic M. A review of epigenetics of PTSD in comorbid psychiatric conditions. Genes (Basel). 2019;10:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lebow MA, Schroeder M, Tsoory M, et al. Glucocorticoid-induced leucine zipper “quantifies” stressors and increases male susceptibility to PTSD. Transl Psychiatry. 2019;9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang Y, Karstoft KI, Nievergelt CM, et al. Post-traumatic stress following military deployment: genetic associations and cross-disorder genetic correlations. J Affect Disord. 2019;252:350-357. [DOI] [PubMed] [Google Scholar]
- 64. Gelernter J, Sun N, Polimanti R, et al. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat Neurosci. 2019;22:1394-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Depue BE, Olson-Madden JH, Smolker HR, Rajamani M, Brenner LA, Banich MT. Reduced amygdala volume is associated with deficits in inhibitory control: a voxel- and surface-based morphometric analysis of comorbid PTSD/mild TBI. Biomed Res Int. 2014;2014:691505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hayes JP, Hayes S, Miller DR, Lafleche G, Logue MW, Verfaellie M. Automated measurement of hippocampal subfields in PTSD: evidence for smaller dentate gyrus volume. J Psychiatr Res. 2017;95:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rabinak CA, Angstadt M, Welsh RC, et al. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry. 2011;2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kennis M, Van Rooij SJ, Van den Heuvel MP, Kahn RS, Geuze E. Functional network topology associated with posttraumatic stress disorder in veterans. Neuroimage Clin. 2016;10:302-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yuan H, Phillips R, Wong CK, et al. Tracking resting state connectivity dynamics in veterans with PTSD. Neuroimage Clin. 2018;19:260-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Badura-Brack A, McDermott TJ, Heinrichs-Graham E, et al. Veterans with PTSD demonstrate amygdala hyperactivity while viewing threatening faces: a MEG study. Biol Psychol. 2018;132:228-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sanjuan PM, Thoma R, Claus ED, Mays N, Caprihan A. Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry Res. 2013;214:260-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aupperle RL, Connolly CG, Stillman AN, May AC, Paulus MP. Deployment and post-deployment experiences in OEF/OIF veterans: relationship to gray matter volume. PLoS ONE. 2013;8:e75880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Averill CL, Averill LA, Wrocklage KM, et al. Altered white matter diffusivity of the cingulum angular bundle in posttraumatic stress disorder. Mol Neuropsychiatry. 2018;4:75-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lindemer ER, Salat DH, Leritz EC, McGlinchey RE, Milberg WP. Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF veterans and the impact of comorbid TBI. Neuroimage Clin. 2013;2:601-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wrocklage KM, Averill LA, Cobb Scott J, et al. Cortical thickness reduction in combat exposed U.S. veterans with and without PTSD. Eur Neuropsychopharmacol. 2017;27:515-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fischer BL, Parsons M, Durgerian S, et al. Neural activation during response inhibition differentiates blast from mechanical causes of mild to moderate traumatic brain injury. J Neurotrauma. 2014;31:169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hayes JP, Miller DR, Lafleche G, Salat DH, Verfaellie M. The nature of white matter abnormalities in blast-related mild traumatic brain injury. Neuroimage Clin. 2015;8:148-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rangaprakash D, Dretsch MN, Venkataraman A, Katz JS, Denney TS, Jr, Deshpande G. Identifying disease foci from static and dynamic effective connectivity networks: illustration in soldiers with trauma. Hum Brain Mapp. 2018;39:264-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Miller DR, Hayes JP, Lafleche G, Salat DH, Verfaellie M. White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Hum Brain Mapp. 2016;37:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Howlett JR, Stein MB. Post-traumatic stress disorder: relationship to traumatic brain injury and approach to treatment. In: Laskowitz D, Grant G. eds. Translational Research in Traumatic Brain Injury. Boca Raton, FL: CRC Press/Taylor and Francis Group; 2015. https://www.ncbi.nlm.nih.gov/books/NBK326723/. [PubMed] [Google Scholar]
- 82. Hoge CW, McGurk D, Thomas J, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358:454-463. [DOI] [PubMed] [Google Scholar]
- 83. Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol. 2008;167:1446-1452. [DOI] [PubMed] [Google Scholar]
- 84. Kontos AP, Kotwal RS, Elbin RJ, et al. Residual effects of combat-related mild traumatic brain injury. J Neurotrauma. 2013;30:680-686. [DOI] [PubMed] [Google Scholar]
- 85. Kennedy JE, Leal FO, Lewis JD, Cullen MA, Amador RR. Posttraumatic stress symptoms in OIF/OEF service members with blast-related and non-blast-related mild TBI. Neurorehabilitation. 2010;26:223-231. [DOI] [PubMed] [Google Scholar]
- 86. Vasterling JJ, Jacob SN, Rasmusson A. Traumatic brain injury and posttraumatic stress disorder: conceptual, diagnostic, and therapeutic considerations in the context of co-occurrence. J Neuropsychiatry Clin Neurosci. 2018;30:91-100. [DOI] [PubMed] [Google Scholar]
- 87. McAllister TW, Stein MB. Effects of psychological and biomechanical trauma on brain and behavior. Ann N Y Acad Sci. 2010;1208:46-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Perez-Garcia G, Gama Sosa MA, De Gasperi R, et al. Blast-induced “PTSD”: evidence from an animal model. Neuropharmacology. 2019;145:220-229. [DOI] [PubMed] [Google Scholar]
- 89. Elder GA, Dorr NP, De Gasperi R, et al. Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J Neurotrauma. 2012;29:2564-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Perez-Garcia G, Gama Sosa MA, De Gasperi R, et al. Chronic post-traumatic stress disorder-related traits in a rat model of low-level blast exposure. Behav Brain Res. 2018;340:117-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Perez-Garcia G, De Gasperi R, Gama Sosa MA, et al. PTSD-related behavioral traits in a rat model of blast-induced mTBI are reversed by the mGluR2/3 receptor antagonist BCI-838. eNeuro. 2018;5:ENEURO.0357-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Elder GA, Stone JR, Ahlers ST. Effects of low-level blast exposure on the nervous system: is there really a controversy? Front Neurol. 2014;5:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Williams JL, McDevitt-Murphy ME, Murphy JG, Crouse EM. Postconcussive symptoms, PTSD, and medical disease burden in treatment-seeking OEF/OIF/OND veterans. Mil Med. 2017;182:e1645-e1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Andrews RJ, Fonda JR, Levin LK, McGlinchey RE, Milberg WP. Comprehensive analysis of the predictors of neurobehavioral symptom reporting in veterans. Neurology. 2018;91:e732-e745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Porter KE, Stein MB, Martis B, et al. Postconcussive symptoms (PCS) following combat-related traumatic brain injury (TBI) in veterans with posttraumatic stress disorder (PTSD): influence of TBI, PTSD, and depression on symptoms measured by the Neurobehavioral Symptom Inventory (NSI). J Psychiatr Res. 2018;102:8-13. [DOI] [PubMed] [Google Scholar]
- 96. Avallone KM, Smith ER, Ma S, et al. PTSD as a mediator in the relationship between post-concussive symptoms and pain among OEF/OIF/OND veterans. Mil Med. 2019;184:e118-e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pietrzak RH, Johnson DC, Goldstein MB, Malley JC, Southwick SM. Posttraumatic stress disorder mediates the relationship between mild traumatic brain injury and health and psychosocial functioning in veterans of Operations Enduring Freedom and Iraqi Freedom. J Nerv Ment Dis. 2009;197:748-753. [DOI] [PubMed] [Google Scholar]
- 98. Lippa SM, Pastorek NJ, Benge JF, Thornton GM. Postconcussive symptoms after blast and nonblast-related mild traumatic brain injuries in Afghanistan and Iraq war veterans. J Int Neuropsychol Soc. 2010;16:856-866. [DOI] [PubMed] [Google Scholar]
- 99. Neipert L, Pastorek NJ, Troyanskaya M, Scheibel RS, Petersen NJ, Levin HS. Effect of clinical characteristics on cognitive performance in service members and veterans with histories of blast-related mild traumatic brain injury. Brain Inj. 2014;28:1667-1674. [DOI] [PubMed] [Google Scholar]
- 100. Nelson NW, Hoelzle JB, Doane BM, et al. Neuropsychological outcomes of U.S. veterans with report of remote blast-related concussion and current psychopathology. J Int Neuropsychol Soc. 2012;18:845-855. [DOI] [PubMed] [Google Scholar]
- 101. Merz ZC, Roskos PT, Gfeller JD, Bucholz RD. Impact of psychiatric symptomatology on neuropsychological assessment performance in persons with TBI: a comparison of OEF/OIF veteran and civilian samples. Brain Inj. 2017;31:1422-1428. [DOI] [PubMed] [Google Scholar]
- 102. Storzbach D, O’Neil ME, Roost SM, et al. Comparing the neuropsychological test performance of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans with and without blast exposure, mild traumatic brain injury, and posttraumatic stress symptoms. J Int Neuropsychol Soc. 2015;21:353-363. [DOI] [PubMed] [Google Scholar]
- 103. Shandera-Ochsner AL, Berry DT, Harp JP, et al. Neuropsychological effects of self-reported deployment-related mild TBI and current PTSD in OEF/OIF veterans. Clin Neuropsychol. 2013;27:881-907. [DOI] [PubMed] [Google Scholar]
- 104. Verfaellie M, Lafleche G, Spiro A, Bousquet K. Neuropsychological outcomes in OEF/OIF veterans with self-report of blast exposure: associations with mental health, but not MTBI. Neuropsychology. 2014;28:337-346. [DOI] [PubMed] [Google Scholar]
- 105. Cooper DB, Curtiss G, Armistead-Jehle P, et al. Neuropsychological performance and subjective symptom reporting in military service members with a history of multiple concussions: comparison with a single concussion, posttraumatic stress disorder, and orthopedic trauma. J Head Trauma Rehabil. 2018;33:81-90. [DOI] [PubMed] [Google Scholar]
- 106. White M, Epston D. Narrative Means to Therapeutic Ends. New York, NY: W.W. Norton & Company; 1990. [Google Scholar]
- 107. McCann IL, Pearlman LA. Psychological Trauma and the Adult Survivor: Theory, Therapy, and Transformation. New York, NY: Taylor & Francis; 1990. [Google Scholar]