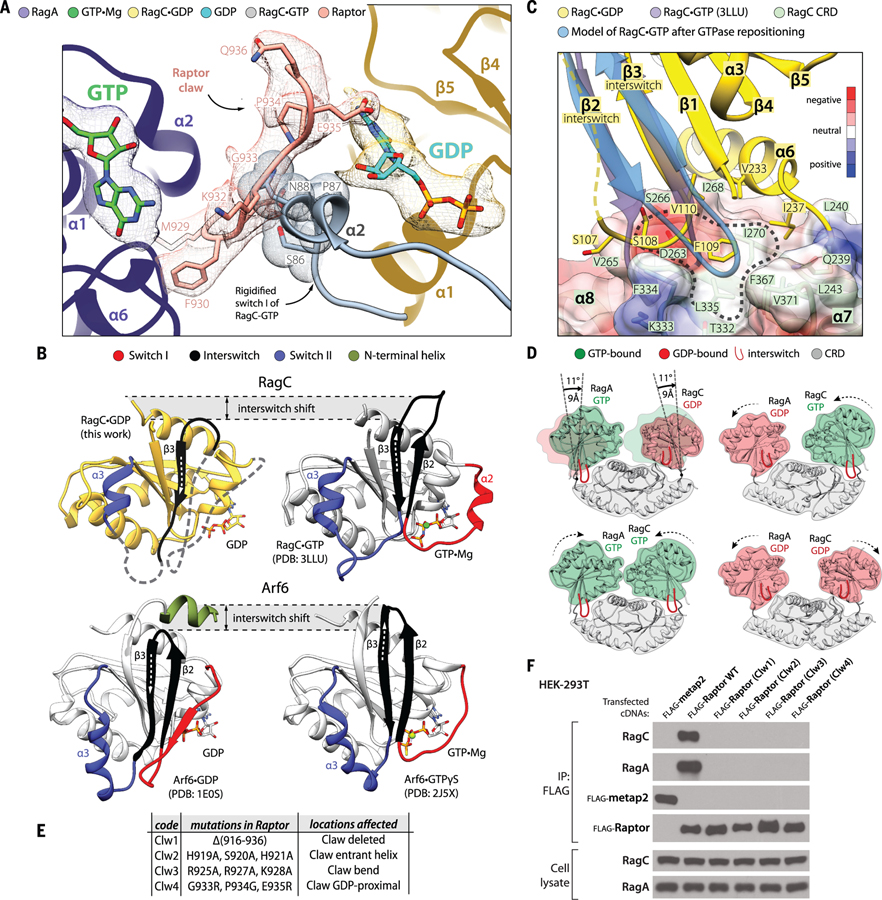
Figure 4. The dynamics of the Rag-Raptor interaction.
(A) Loading of GTP by RagC would trigger its switch I to rigidify and clash with the Raptor claw. The structure of the GTP-loaded GTPase domain of RagC (PDB ID 3LLU) was superimposed with our cryo-EM structure of RagC•GDP. (B) The organization of the switches in RagC (top) and Arf6 (bottom) [PDB ID: 1E0S, 2J5X (37, 38)]. Note that although the switches change their positions in GTP- versus GDP-loaded states, the core of the structure does not move. Even though a large proportion of the switches in our GDP-loaded RagC structure are disordered, we observed that the interswitch changes its register by two residues, in a manner similar to Arf GTPases. (C) The movement of the interswitch during the GDP-to-GTP transition would cause its loop to clash with the CRD pocket (circled area). Instead, the interswitch repositions itself such that it engages with the more central part of the CRD. The disordered interswitch strand β2 of RagC•GDP is drawn with a dashed line. The surface of the CRD is colored according to electrostatic potential (see the color key). (D) The shifting of the GTPase domains in the Rag heterodimer during GTP-GDP binding exchanges. GTPase domains loaded with GDP are positioned away from the central axis of the Rag heterodimer, and their interswitches are retracted. Loading of GTP causes the interswitch to extend and press the CRD pocket such that the entire GTPase domain becomes repositioned closer to the Rag central axis. The models were created by superimposing RagA•GTP with RagC•GDP through their CRDs (specifically by matching their β7 and α9). (E) Description of the Raptor claw mutants used in (F). (F) Elimination of the Raptor claw or mutations in its critical Rag-interacting regions prevent Raptor from coimmunoprecipitating the RagA-RagC heterodimer. Longer exposures of the RagA and RagC immunoblots did not reveal any signal for Raptor. Flag-metap2 was used as a negative control protein.