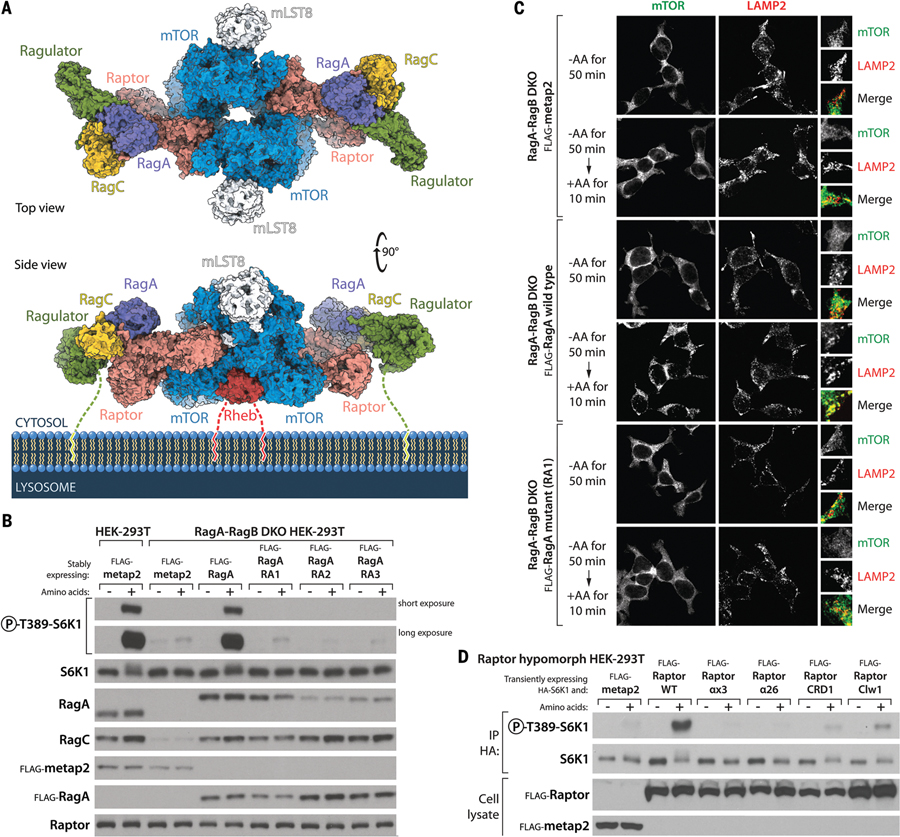
Figure 5. mTORC1 on the lysosomal surface.
(A) Model of a Rheb-activated mTORC1 dimer bound to two lysosomal-targeting Rag-Ragulator complexes. The Rag-Ragulator complex clamps mTORC1 to the surface of the lysosome. Note that both Ragulator and Rheb carry lipid-modified terminal residues that tether the supercomplex to the membrane. Our cryo-EM structure of the Raptor-Rag-Ragulator complex was superimposed with that of Rheb-bound mTORC1 [PDB ID 6BCU (31)]. (B) Stable expression in RagA-RagB DKO HEK-293T cells of RagA mutants that cannot bind Raptor (Fig. 2, D and E) does not restore mTORC1 activity, as assayed by the phosphorylation of S6K1 in amino acid–replete cells. Note that although the levels of the stably expressed wild-type and mutant Flag-RagA are relatively even as assessed with the anti-Flag antibody, the recognition of the RA2 and RA3 RagA mutants by the RagA antibody (monoclonal; CST D8B5) is reduced, which suggests that the mutated residues affect the epitope recognized by this antibody, which has not been disclosed. (C) Unlike expression of wild-type RagA, expression in RagA-RagB DKO HEK-293T cells of the Y31A RagA (RA1) mutant that cannot bind Raptor does not rescue the amino acid–induced colocalization of mTOR with lysosomes, as marked by the lysosomal protein LAMP2. (D) In cells hypomorphic for Raptor, transient expression of wild-type Raptor promotes S6K1 phosphorylation, whereas that of Raptor mutants defective in the RagA-Raptor interaction (αx3 and α26) does not. Mutants that cannot bind the CRD of RagC (CRD1) or lacking the claw (Clw1) activate S6K1 phosphorylation to a small degree. See supplementary materials and fig. S6B for a description of the Raptor hypomorph cell line.