Figure 5.
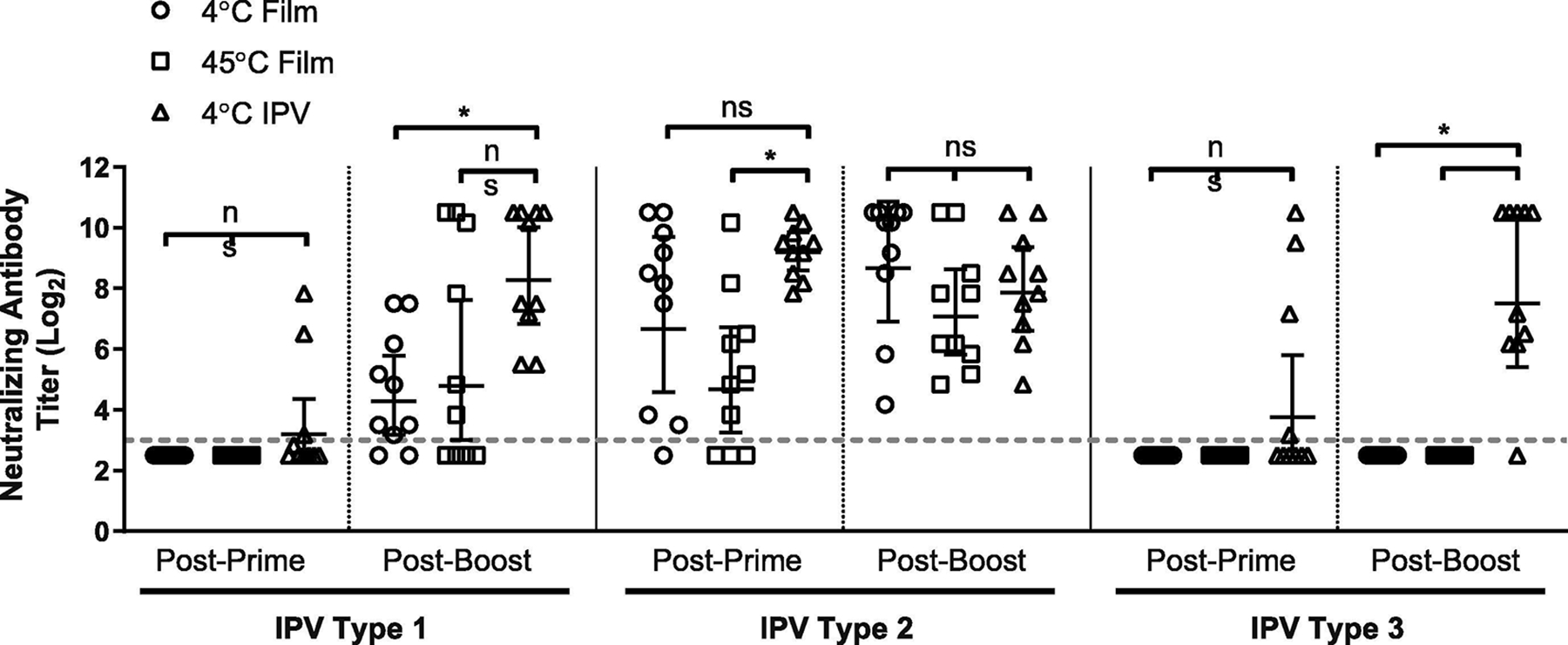
Immunogenicity of IPV antigens stored in thin silk fibroin film format. Formulated IPV films were stored for two weeks at either an elevated temperature (45°C) or refrigerated (4°C) prior to reconstitution and injection to Wistar rats (n=10) to assess retained immunogenicity. IPV (IPOL®, Sanofi-Pasteur, Swiftwater PA) was stored refrigerated and used as an unmodified liquid control for immunization. Neutralizing antibody titers were assessed via micro-neutralization assay on serum collected 4 weeks after primary immunization (day 28, “Post-Prime”) and 4 weeks after booster immunization (day 56, “Post-Boost”). Dashed line represents neutralization titer threshold for seroconversion; geometric means and 95% confidence interval displayed. Significance (ns: not significant, *: p<0.05) was determined using unpaired, two-tailed Student’s T-test.
