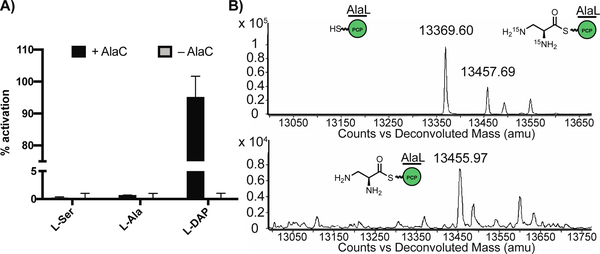
Figure 5.
AlaC selectively activates L-Dap for loading onto AlaL. A) ATP-32PPi exchange assay shows that AlaC preferentially activates L-Dap over other amino acids from 100 μL incubations of N-His6-AlaC (1 μM) with 5 mM dithiothreitol, 5 mM ATP, 1 mM of amino acid substrate, and 4 mM Na4PPi/[32P]PPi in reaction buffer (50 mM HEPES, 200 mM NaCl, 10 mM MgCl2, pH = 8) at room temperature for 30 min. Data are mean ± s.d. of two biological replicates. B) Deconvoluted whole protein mass spectra (ESI+) showing holo-N-His6-AlaL-C-His6 (13369.60) is loaded with 15N2-L-Dap (13457.69) or L-Dap (13455.97). 50 μL reaction mixtures were set up with coenzyme A (1 mM), Sfp (5 μM), and N-His6-AlaL-C-His6 (20 μM) in a solution of reaction buffer (50 mM HEPES, 200 mM NaCl, 10 mM MgCl2, pH = 8). After incubation at room temperature for 2 h, AlaC (20 μM) and L-Dap or L-15N2-Dap (250 μM) were added, followed by ATP (5 mM) to initiate the reaction. Incubated for 1 h at room temperature.