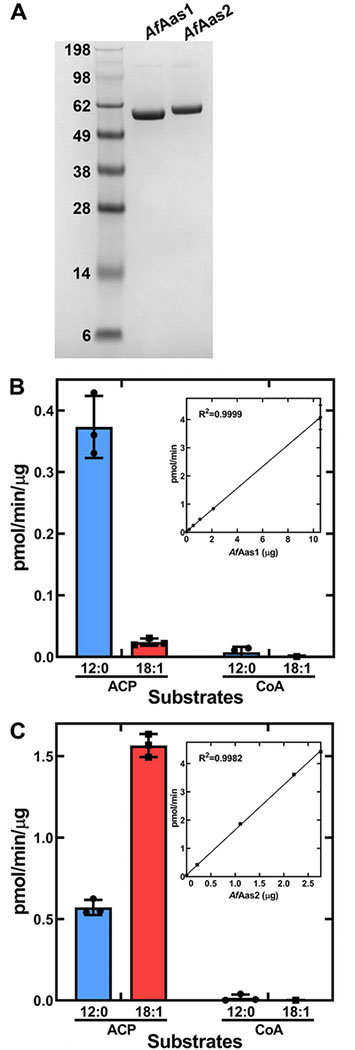
Figure 10. Biochemical analysis of purified AfAas1 and AfAas2.
A. A Coomassie-stained SDS gel electrophoresis illustrating the purity of AfAas1 and AfAas2 used in the biochemical assays to determine the acyl acceptor (ACP or CoA) and acyl donor (12:0 or 18:1) specificities of the two enzymes.
B. Biochemical activity of AfAas1. AfAas1 activity using [14C]12:0 as substrate was linear with protein and had a specific activity of 0.38 ± 0.002 pmol/min/μg protein (Inset).
C. Biochemical activity of AfAas2. AfAas2 activity using [14C]18:1 as substrate was linear with protein and had a specific activity of 1.59 ± 0.02 pmol/min/μg protein (Inset). AfAas2 activity using [14C]12:0 as substrate was 0.57 ± 0.03 pmol/min/μg protein.