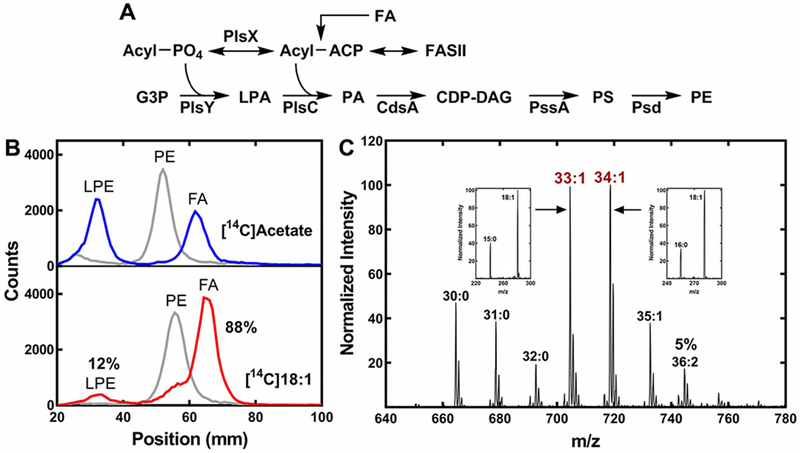
Figure 7. Exogenous oleate incorporation by A. finegoldii.
A. Pathway for the synthesis of PE in A. finegoldii. Acyl-ACP is formed from fatty acid synthesis (FASII) or by the activation of an exogenous fatty acid (FA). The acyl-ACP is converted to acyl-PO4 by PlsX, and the 1-position of glycerol-phosphate (G3P) is acylated by PlsY to form 1-acyl-sn-glycero-3-phosphate (LPA). PlsC acylates the 2-position with an acyl-ACP to form phosphatidic acid (PA) that is converted to CDP-dacylglycerol (CDP-DAG) by CdsA. PssA converts PA to phosphatidylserine (PS), which is then decarboxylated by Psd to form PE.
B. A. finegoldii was metabolically labeled with either [14C]acetate or [14C]oleate and the [14C]PE was isolated by thin-layer chromatography, then digested with phospholipase A2 to liberate the fatty acid at the 2-position. The products, fatty acid (FA) and 1-acyl-glycerophosphoethanolamine (LPE), were separated by thin-layer chromatography and the radioactive products were localized and quantified using a Bioscan Counter. Gray trace, [14C]PE starting material; blue trace, digestion of [14C]PE from [14C]acetate-labeled cells; red trace, digestion of [14C]PE derived from [14C]oleate-labeled cells (18:1). 88% of the [14C]oleate label was incorporated in the 2-position, demonstrating the preference for this position.
C. Representative mass spectrum of PE molecular species in cells grown with 20 μM 18:1. Insets, the fatty acids present in the 33:1 and 34:1 molecular species was determined by MS/MS. The 36:2 molecular species was 5% of the total ion current.