Figure 9. Analysis of the acyl-ACP synthetases of A. finegoldii.
E. coli strain LCH30 (aas fadD) was transformed with a series of expression vectors directing the synthesis of 6xHis-tagged versions of the three Alistipes genes encoding putative synthetases, along with two controls expressing Neisseria acyl-ACP synthetase (NgAas) or E. coli acyl-CoA synthetase (EcFadD). Cells were grown to mid-log phase and either metabolically labeled or extracted to biochemically characterize the expressed enzymes.
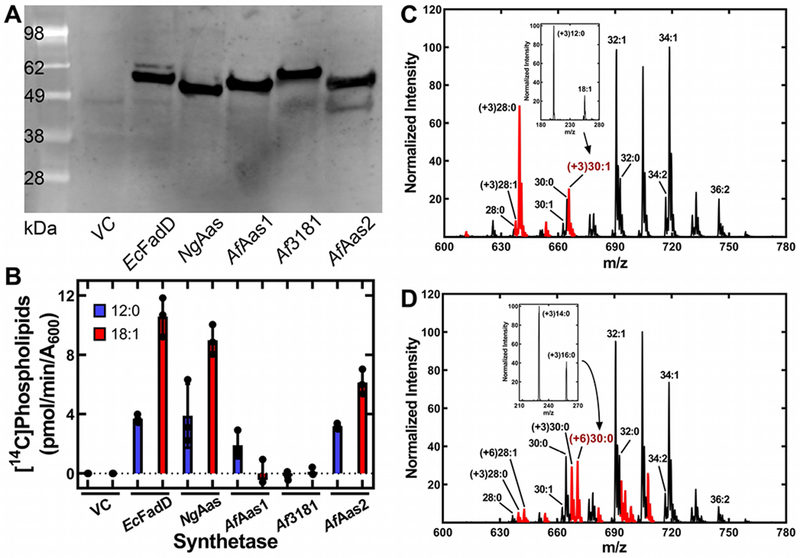
A. Western blot of the expressed proteins using anti-His-tag antibody for detection to validate expression of the synthetases.
B. Metabolic labeling of the strain set with either [14C]12:0 or [14C]18:1 fatty acids. Lipids were extracted, and the amount of radioactivity incorporated into phospholipids determined by scintillation counting.
C. Mass spectrum of PE synthesized by strain LCH30 complemented with EcFadD and labeled with [d3]12:0. Inset, fragmentation of the (+3)30:1 molecular species showing [d3]12:0 was not elongated.
D. Mass spectrum of strain LCH30 complemented with AfAas1 and labeled with [d3]12:0. Inset, fragmentation of the (+6)30:0 PE molecular species showing the presence of [d3]14:0 and [d3]16:0, demonstrating [d3]12:0 was elongated. This spectrum is representative of those obtained from cells complemented with either NgAas or AfAas2 and labeled with [d3]12:0. PE molecular species (C, D) containing a deuterium label are indicated in red.