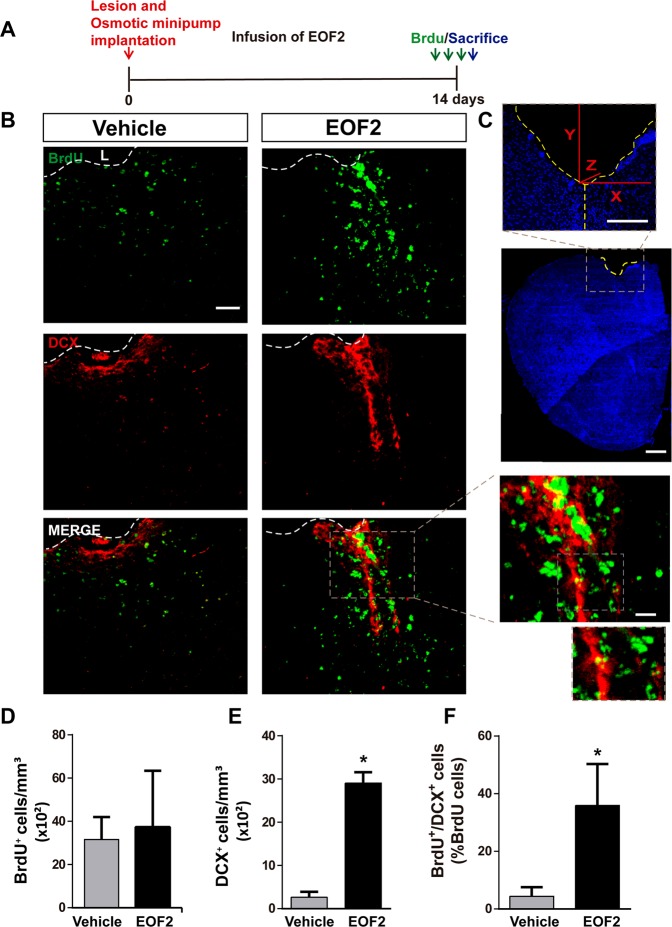
Fig. 5. Local administration of EOF2 induces neuronal differentiation in the injured cortex.
a Scheme of experimental procedures. Mechanical cortical lesions were unilaterally performed in the primary motor cortex of adult mice, and osmotic minipumps were implanted to locally deliver vehicle or EOF2 (5 µM) for 14 days. All mice were intraperitoneally-injected with BrdU on the last day of treatment as described in methods. b Representative confocal microphotographs of the area surrounding cortical lesion in mice brain showing immnunodetection for BrdU (upper panels) Doublecortin (DCX; medium panels) and the merged signals (lower panels). Scale bar represent 50 µm in low magnification images and 20 µm in high magnification images. The dotted line indicates the limit of the lesion (L). c Microphotograph showing details about the injury and the lesion area. In each section, the positive cells labeled with the different markers were quantified in the peri-lesional area that corresponds to 200 µm-wide band of tissue surrounding the lesion border. Scale bar represent 1 mm in low magnification images and 200 µm in high magnification images. d Graph shows the number of proliferating cells labeled with BrdU per mm3 in the peri-lesional area of the indicated animal groups. Data shown are the mean ± S.E.M.; n = 6 animals per group. e Quantification of DCX+ (doublecortin) cells per mm3 in the peri-lesional area of the indicated animal groups. Data shown are the mean ± S.E.M.; n = 6 animals per group. Statistical analysis: *p < 0.0001 in two tailed unpaired Student’s t test comparing EOF2 with the control. f Graph shows the percentage of BrdU+ cells that co-express the neuronal marker DCX in the peri-lesional area of the indicated animal groups. Data shown are the mean ± S.E.M.; n = 6 animals per group. Statistical analysis: *p = 0.0320 in two tailed unpaired Student’s t test comparing EOF2 with the control.