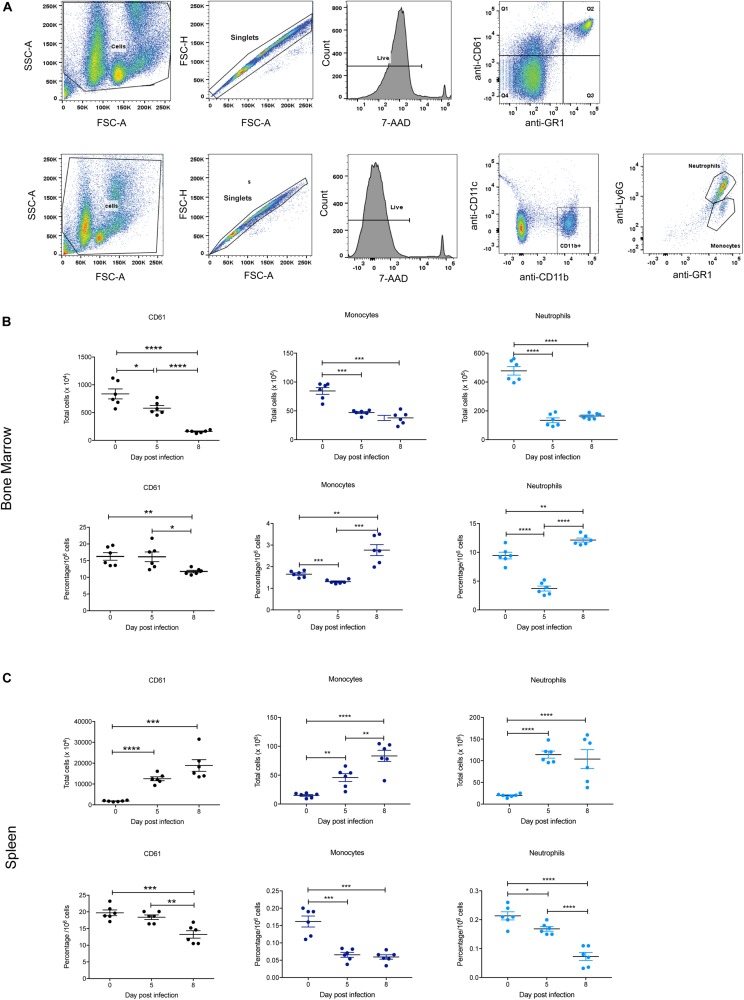
FIGURE 6.
Impact of P. berghei infection on megakaryocyte, neutrophil, and monocyte populations in the bone marrow and spleen. Gating strategy for identifying megakaryocytic, monocyte, and neutrophil cells (A). For megakaryocytic cells (top panel), cells were firstly gated on size and granularity based on forward and side scatter to remove debris. FSC-H and FSC-A plots were then used to identify singlets. Only live cells, which are negative for 7-AAD staining were analyzed for CD61 and GR1 staining. Cells positive for CD61 but negative for Gr1 (i.e., those in Q1) were gated and analyzed. Shown is example of cells isolated from the bone marrow of uninfected Balb/c mice. Gating strategy for monocytes and neutrophils (bottom panel). Cells were gated on size and granularity based on forward and side scatter to remove debris. After selection of singlets and live cells, cells were analyzed for CD11c and CDl1b staining. Cells positive for CD11b but negative for CD11c were gated on to remove dendritic cells and the resulting cell population was analyzed for Gr1 and Ly6G expression to distinguish between neutrophils (Gr-1+, Ly6G+) and monocytes (Gr-1+, Ly6G–). Shown is example of cells isolated from the bone marrow of uninfected Balb/c mice. CD61+ megakaryocytes, CD11b+Gr1+Ly6G– monocytes and CD11b+Gr1+Ly6G+ neutrophils in the bone marrow (B) and spleen (C) during the course of a P. berghei infection were then examined, from which the total number (top panel) and proportion of cells (lower panel) were calculated. Plotted are the results from individual mice as well as mean ± SD (n = 6 mice). An unpaired t-test was used to calculate statistical significance between two groups: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.