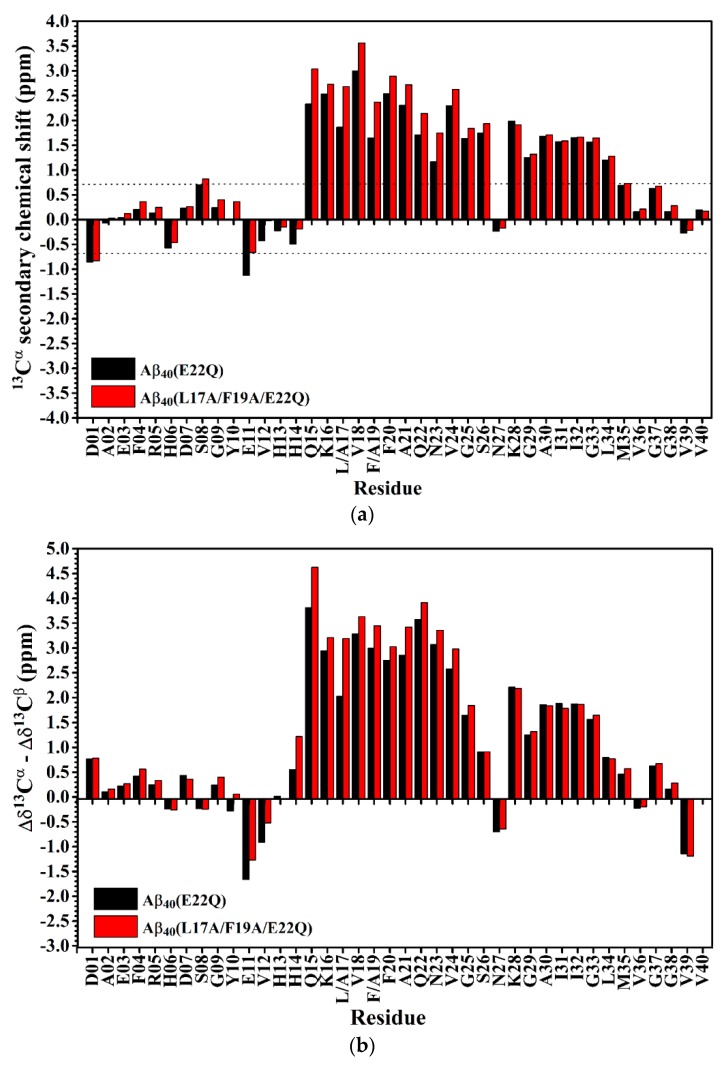
Figure 6.
(a) 13Cα secondary chemical shifts of Aβ40(E22Q) (black) and Aβ40(L17A/F19A/E22Q) (red) plotted as a function of residue. In principle, if the 13Cα secondary chemical shift of an amino acid residue is greater than 0.7 ppm, its conformation would be α-helical [35]; (b) Differences between Δδ13Cα (13Cα secondary chemical shift) and Δδ13Cβ (13Cβ secondary chemical shift) of Aβ40(E22Q) (black) and Aβ40(L17A/F19A/E22Q) (red) plotted as a function of residue. Δδ13Cα (or Δδ13Cβ) was defined as the difference between the observed 13Cα (or 13Cβ) chemical shift of an amino acid residue and its 13Cα (or 13Cβ) chemical shift in a random coil conformation. If Δδ13Cα–Δδ13Cβ for an amino acid residue is positive, its conformation would be α-helical. For a more detailed description of the relationship between the value of Δδ13Cα–Δδ13Cβ and secondary structure of an amino acid residue please see the reference [34].