Abstract
Objectives
We implemented the WHO cross-sectional survey protocol to determine rates of HIV viral load (VL) suppression (VLS), and weighted prevalence, predictors and patterns of acquired drug resistance (ADR) in individuals with virological failure (VF) defined as VL ≥1000 copies/mL.
Methods
We enrolled 547 and 1064 adult participants on first-line ART for 12 (±3) months (ADR12) and ≥48 months (ADR48), respectively. Dried blood spots and plasma specimens were collected for VL testing and genotyping among the VFs.
Results
VLS was 95.0% (95% CI 93.4%–96.5%) in the ADR12 group and 87.9% (95% CI 85.0%–90.9%) in the ADR48 group. The weighted prevalence of ADR was 96.1% (95% CI 72.9%–99.6%) in the ADR12 and 90.4% (95% CI 73.6–96.8%) in the ADR48 group, out of the 30 and 95 successful genotypes in the respective groups. Initiation on a zidovudine-based regimen compared with a tenofovir-based regimen was significantly associated with VF in the ADR48 group; adjusted OR (AOR) 1.96 (95% CI 1.13–3.39). Independent predictors of ADR in the ADR48 group were initiation on a zidovudine-based regimen compared with tenofovir-based regimens, AOR 3.16 (95% CI 1.34–7.46) and ART duration of ≥82 months compared with <82 months, AOR 1.92 (95% CI 1.03–3.59).
Conclusions
While good VLS was observed, the high prevalence of ADR among the VFs before they underwent the recommended three intensive adherence counselling (IAC) sessions followed by repeat VL testing implies that IAC prior to treatment switching may be of limited benefit in improving VLS.
Introduction
The Joint United Nations Programme on HIV/AIDS (UNAIDS) set up the 90–90–90 targets that aim to have 90% of people living with HIV knowing their status, 90% of these on ART and 90% of those on ART virally suppressed.1 The ART programmes in resource-limited settings are characterized by the use of limited antiretroviral regimens and often limited use of viral load (VL) testing to monitor treatment outcomes and to maximize the long-term effectiveness of ART. To ensure sustainability of ART programmes, the WHO has recommended HIV drug resistance (HIVDR) monitoring and surveillance.2 Even in settings with optimal ART programme management, some degree of HIVDR is expected to emerge in populations receiving ART, which could affect the response to ART, as well as be a source of HIVDR transmission.
In the WHO HIVDR monitoring and surveillance strategy, surveillance of HIVDR in adult populations both initiating3 and receiving ART4 has been recommended, with guidance for similar surveillance in children. HIVDR surveillance in nationally representative populations initiating first-line ART regimens is essential to inform the selection of first-line ART combinations, while surveillance of HIVDR in populations receiving first-line ART is critical to assess ART programme quality and inform the selection of second-line ART regimens. Suboptimal VL suppression (VLS) and the detection of HIVDR in populations receiving ART may reflect gaps in ART programme quality, including inadequate adherence assessment and support, interruptions in drug supply and low retention in care.
The earliest surveys conducted in Uganda indicated that the prevalence of HIVDR varied across populations: <5% for transmitted drug resistance among pregnant women in Entebbe;5 2.6% among sex workers in Kampala;6 1.4% in a rural population;7 6% among individuals with recent HIV infection in Masaka and Entebbe;8 and 8.6% among young adults in Kampala.9 There are a few studies conducted for pre-treatment and acquired HIVDR (ADR) among adults in sub-Saharan Africa; in one multicentre study in five African countries including three sites in Uganda, the pooled pre-treatment/baseline HIVDR prevalence for Ugandan sites was 11.6%.10 In another prospective survey conducted among 420 adults in Uganda from 2012 to 2013, the baseline prevalence of HIVDR was 4.9% and increased to 9.0% after 12 months of ART.11 Another study reported HIVDR prevalence of 7% after 6 months of ART in a cohort of 325 patients in central Uganda.12 A recent meta-analysis has shown a substantial increase of pre-treatment NNRTI drug resistance in low- and middle-income countries especially in sub-Saharan Africa,13 while another study in Uganda showed an increasing prevalence of pre-treatment drug resistance in women but not among men in the period between 2005 and 2013.14 In this report, we present findings using the WHO cross-sectional survey protocol of HIVDR among adults receiving ART.4 We report levels of VLS among adult patients on first-line ART for 12 (±3) and ≥48 months, and we determined the predictors of virological failure (VF; defined as VL ≥1000 copies/mL) and the weighted prevalence and patterns of ADR mutations in patients with VF.
Methods
Study setting and participants
This survey was designed and the analyses conducted following the WHO methods for surveillance of HIVDR in adults receiving ART.4 This was a cross-sectional survey conducted using a two-stage cluster design among adult (≥18 years) populations receiving ART for 12 (±3) months (ADR12) at 23 out of the 24 selected sites; and among those receiving ART for at least 48 months (ADR48) at the 24 sites. Clinics chosen for the ADR12 survey were chosen among those that had been dispensing ART for at least 15 months and those chosen for the ADR48 survey were selected among clinics that had been dispensing ART for at least 48 months. The main purpose of this survey was to determine a nationally representative prevalence estimate with associated confidence intervals of VLS and presence of HIVDR by genotypic testing among the VFs. We also determined independent predictors of VF and prevalence and patterns of HIVDR in these two populations. The study populations included all HIV-infected persons who provided written informed consent. Patients whose date of ART initiation was unknown were excluded. Eligible participants were enrolled sequentially until the desired sample size for each site was reached. Consenting participants were assigned a unique study identifier, and study variables were abstracted from each patient file.
Site and patient selection for ADR12 and ADR48
The ADR12 survey was conducted in August to November 2016 while the ADR48 survey was done in September to November 2017. Prior to sampling, study sites were selected from a list of all clinics in the country dispensing ART for at least 12 months and 48 months, for the ADR12 and ADR48 surveys, respectively. The smallest sites that made up <10% of the total population of patients on ART were excluded. The clinics were then stratified into five geographical regions and sites were randomly selected proportionally to the size of the region. Considering the prevalence of VLS of 85% at 12 months and 70% at 48 months, a PCR amplification rate of 80%, an intracluster correlation coefficient for VLS of 0.8%, a design effect of 1.5 and accounting for dropout, 24 sites with 22 participants and 24 sites with 46 patients from each clinic were chosen for ADR12 and ADR48 surveys, respectively.
A base weight for each sampled unit was then constructed to correct for their unequal probability of selection. The base weight was constructed by taking the reciprocal of its probability of selection into the sample. This being a two-stage design (sites at the regional level and individuals at the site level), the base weights reflected probabilities of selection at each stage.
VL testing and HIVDR genotyping
Venous whole blood was collected for preparation of two dry blood spot (DBS) cards containing five spots each or two aliquots of plasma specimens15,16 from each patient, with one DBS card or plasma aliquot for VL testing and the second card or plasma aliquot for genotypic testing. The DBS specimens were shipped at room temperature from the sites to Central Public Health Laboratories (CPHLs) using the National Sample and Results Transport Network17 also used by the Early Infant Diagnosis programme.18 Plasma specimens were transported in liquid nitrogen tanks to CPHLs. VL testing was done at CPHLs using a DBS-VL protocol using the Abbott RealTime HIV-1 Assay Kit (Abbott Molecular Diagnostics, Des Plaines, IL, USA) and plasma using COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test, v2.0 kit (Roche Molecular System, Inc., Pleasanton, CA, USA). All specimens tested and found with VL ≥1000 copies/mL were shipped to the MRC/Uganda Virus Research Institute (UVRI) and London School of Hygiene and Tropical Medicine (LSHTM) Uganda Research Unit laboratories for genotyping and analysis for HIVDR mutations (HIVDRMs).
Genotyping of the protease and reverse transcriptase regions of the HIV-1 pol gene was done using a modified and validated in-house method11,16 at the MRC/UVRI and LSHTM laboratory, which is a WHO HIVDR Network designated reference laboratory. The 1300 bp segment of the 5′ region of the pol gene was generated by RT–PCR followed by nested PCR using total nucleic acid extracted from the DBS or nucleic acid from plasma. The fragment was purified, sequenced using a BigDye Terminator v3.1 cycle sequencing kit (Life Technologies, Foster City, CA, USA) and analysed on an ABI Prism 3500 Genetic Analyzer (Life Technologies). A combination of the Sequencher software (Sequencher® version 5.4.1 sequence analysis software, Gene Codes Corporation, Ann Arbor, MI, USA; http://www.genecodes.com) and a customized RECall software program19 was used to edit the raw sequence data and generate consensus sequences. Drug resistance mutations (DRMs) were analysed by the Stanford HIVdb Program using the 2009 WHO mutation list.20 For quality control purposes, our laboratory is enrolled in the Virology Quality Assurance programme and all sequences generated in the laboratory are assessed for cross-contamination by phylogenetic analysis. Sequences from this study were deposited in GenBank under accession numbers MK359486 to MK359634.
Data management and statistical methods
The patient data were entered into a Microsoft Access database. Laboratory data comprising VL and HIVDR results were linked to clinical data using the patient study identifier. All statistical analyses were performed using STATA version 12 (StataCorp LP, College Station, TX, USA). VF and HIVDR weighted prevalence rates with 95% CIs were calculated. Statistical analysis for weighted prevalence of VLS and HIVDR accounted for the survey design features which included stratification, clustering and survey weights. Weights accounted for unequal selection probabilities, for both selection of clinics and selection of patients. Stratification was done to reduce sampling variation to account for stratification by geographical region during selection of clinics. Clustering accounted for the two clustering units (patients and clinics). Survey logistic regression was used to determine associations between patient characteristics and the outcomes: VF and HIVDR at the P < 0.05 level of significance. Variables that had a P value <0.20 in simple analysis were included in the adjusted logistic regression model. However, age and gender, which are potential confounders, were added to the models irrespective of their P value at univariate analysis.21 Adjusted ORs (AORs), 95% CIs and P values were calculated. By using the svyset function, the analysis adjusted for survey weights, clustering and stratification when generating prevalence rates and ORs.
Ethics
This study was approved by the UVRI Research and Ethics Committee [UVRI-REC Federal Wide Assurance (FWA) No. 00001354] and the Uganda National Council for Science and Technology (FWA No. 00001293). The study was also reviewed in accordance with the CDC human research protection procedures and determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes. All participants gave their written informed consent prior to enrolment into the study.
Results
The ADR12 survey
Enrolment profile, and socio-demographic and clinical characteristics
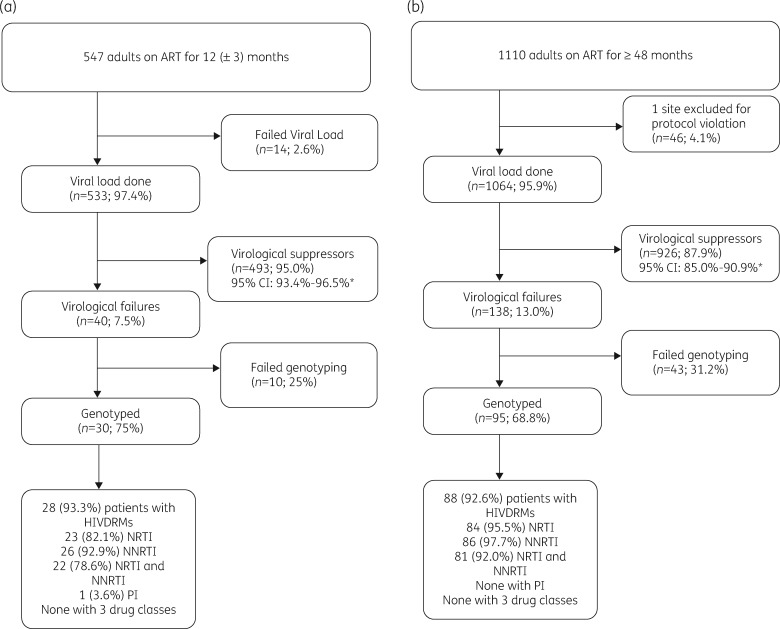
During the study period, a total of 547 patients were enrolled from 23 of the 24 selected sites distributed across the country (Figure 1) between August and November 2016. One site was excluded because they declined to participate in the pre-survey training. A majority were female (365; 66.7%), with an overall median age of 34 years (IQR 28–41), median CD4 count at ART initiation of 318 cells/mm3 (IQR 182–450) and 81.9% were on the recommended tenofovir (TDF) + lamivudine (3TC) + efavirenz (EFV, n = 439)/nevirapine (NVP, n = 9) first-line regimen at that time (Table 1); 39 (7.1%) patients were on TDF, emtricitabine (FTC) and EFV, and 60 (11.0%) patients were on zidovudine (ZDV), 3TC and EFV, n = 26 or NVP, n = 34.
Figure 1.
Enrolment profiles for ADR12 (August to November 2016) (a) and ADR48 (September to November 2017) (b) surveys of HIV-positive adults from the selected sites in Uganda. The asterisk indicates weighted analysis.
Table 1.
Demographic, clinical and immunological characteristics of the 547 ADR12 participants in Uganda
| Characteristics | Total |
|---|---|
| Gender | |
| male | 182 (33.3) |
| female | 365 (66.7) |
| Age, years, median (IQR) | 34 (28–41) |
| Region | |
| Central | 166 (30.3) |
| East | 69 (12.6) |
| Kampala | 34 (6.2) |
| North | 96 (17.6) |
| West | 182 (33.3) |
| CD4 count at ART initiation, cells/mm3, median (IQR) | 318 (182–450) |
| Viral loada | |
| <1000 copies/mL | 493 (92.5) |
| ≥1000 copies/mL | 40 (7.5) |
| VL among VFs, log10 copies/mL, median (IQR) | 4.6 (4.2–4.8) |
| Time on ART, months, median (IQR) | 12.9 (11.2–14.5) |
| Initial ART regimen | |
| TDF+3TC+EFV/NVP | 448 (81.9) |
| ZDV+3TC+EFV/NVP | 60 (11.0) |
| TDF+FTC+EFV | 39 (7.1) |
Values are n (%) unless otherwise stated.
Fourteen viral loads did not amplify.
HIV VL and DRMs at ADR12
The prevalence of VLS among the 533 patients with VL results was 95.0% (95% CI 93.4%–96.5%) (Figure 1 and Table 2) and the median VL among the 40 VFs was 4.6 log10 copies/mL (95% CI 4.2–4.8) (Table 1). The overall prevalence of HIVDR was 4.7% (95% CI 3.3–6.1) as shown in Table 2. However, 28 of the 30 (96.1%; 95% CI 72.9%–99.6%) patients with VL ≥1000 copies/mL who were successfully genotyped had at least one DRM. One patient (1.1%; 95% CI 0.14%–3.6%) had the I85V PI mutation, 23 (90.3%; 95% CI 65.6%–97.9%) had NRTI mutations, 26 (94.1%; 95% CI 74.3%–98.9%) had NNRTI mutations and 22 (89.5%; 95% CI 64.7%–97.5%) had both NRTI and NNRTI mutations (Table 2). No patient was found with all three drug class mutations. Of the 28 patients with mutations, 82.1% had M184V/I, 75% had K103N/S, 28.6% had K65R mutations, 32% had thymidine analogue mutations (TAMs) and 64.3% of participants had the M184V/I NRTI mutation. Table S1 (available as Supplementary data at JAC Online) summarizes the mutations among the 28 participants.
Table 2.
Weighted prevalencea of virological suppression and HIVDR among ADR12 participants
| Survey outcome | n/N | Percentage prevalence (95% CI) |
|---|---|---|
| VL suppression | ||
| all | 493/533 | 95.0 (93.4–96.5) |
| male | 169/182 | 97.7 (91.3–99.4)b |
| female | 324/351 | 93.6 (91.9–95.2) |
| agec <34 years | 267/287 | 94.8 (91.1–98.5) |
| age ≥34 years | 225/245 | 95.1 (91.3–98.9) |
| HIVDR | ||
| any | 28/533 | 4.7 (3.3–6.1) |
| NRTI | 23/533 | 4.4 (3.1–5.8) |
| NNRTI | 26/533 | 4.6 (3.3–6.0) |
| NRTI+NNRTI | 22/533 | 4.4 (3.0–5.8) |
| PI | 1/533 | 0.1 (0.01–0.2) |
| HIVDR among VFs | ||
| any | 28/30 | 96.1 (72.9–99.6)b |
| NRTI | 23/30 | 90.3 (65.6–97.9)b |
| NNRTI | 26/30 | 94.1 (74.3–98.9)b |
| NRTI+NNRTI | 22/30 | 89.5 (64.7–97.5)b |
| PI | 1/30 | 1.1 (0.14–3.6) |
All prevalences are weighted and accounted for the two-stage clustered survey design.
Log-transformed CI.
One missing age.
Factors associated with VF and HIVDRMs at ADR12
There were no factors found to be significantly associated with VF and HIVDRMs among patients receiving ART for 12 (± 3) months (Tables 3 and 4).
Table 3.
Independent factors associated with virological failure among the 40 ADR12 participants in Uganda
| Univariate analysis |
Multivariate analysisa |
||||
|---|---|---|---|---|---|
| Characteristics | VL ≥1000 copies/mL, n (%)b | crude OR (95% CI) | P value | adjusted OR (95% CI) | P value |
| Gender | |||||
| male | 13 (7.1) | 1 | 1 | ||
| female | 27 (7.7) | 2.87 (0.69–11.79) | 0.14 | 7.36 (0.84–644.7) | 0.33 |
| Age | |||||
| ≥34 years | 20 (7.0) | 1 | 1 | ||
| <34 years | 20 (8.2) | 1.08 (0.26–4.54) | 0.91 | 0.21 (0.00–22.32) | 0.46 |
| Region | 0.002 | ||||
| Central | 13 (8.0) | 1 | 1 | ||
| East | 10 (14.7) | 1.61 (0.43–5.96) | 0.46 | 1.06 (0.26–4.33) | 0.91 |
| Kampala | 4 (11.8) | 2.35 (1.41–3.91) | 0.002 | – | – |
| North | 3 (3.2) | 1.17 (0.43–3.17) | 0.74 | 1.25 (0.69–2.27) | 0.40 |
| West | 10 (5.7) | 0.76 (0.37–1.53) | 0.42 | 0.97 (0.03–34.95) | 0.99 |
| CD4 count at ART initiationc | |||||
| ≥250 cells/mm3 | 7 (5.6) | 1 | 1 | ||
| <250 cells/mm3 | 12 (15.8) | 3.85 (0.67–22.14) | 0.11 | 0.99 (0.99–1.00) | 0.18 |
| Initial ART regimen | 0.72 | ||||
| TDF+3TC+EFV/NVP | 34 (7.8) | 1 | |||
| ZDV+3TC+EFV/NVP | 5 (8.6) | 0.82 (0.19–3.49) | 0.77 | ||
| TDF+FTC+EFV | 1 (2.6) | 2.18 (0.31–15.40) | 0.41 | ||
Age and gender were added to the multivariate model to control for confounding.
Fourteen patients whose VL did not amplify were not included in the analysis.
CD4 counts of 21 patients were missing.
Table 4.
Predictors of HIVDR in the 28 ADR12 participants receiving ART in Uganda
| Univariate analysis |
Multivariate analysisa |
||||
|---|---|---|---|---|---|
| Characteristics | HIVDRMs, n (%) | crude OR (95% CI) | P value | adjusted OR (95% CI) | P value |
| Gender | |||||
| male | 8 (4.4) | 1 | 1 | ||
| female | 20 (5.7) | 2.15 (0.31–14.75) | 0.41 | 8.34 (0.04–1839.12) | 0.38 |
| Age | |||||
| ≥34 years | 15 (5.2) | 1 | 1 | ||
| <34 years | 13 (5.3) | 0.79 (0.16–3.78) | 0.91 | 0.07 (0.00–8.20) | 0.23 |
| Region | 0.002 | ||||
| Central | 10 (6.2) | 1 | 1 | ||
| East | 8 (11.8) | 0.96 (0.08–11.27) | 0.97 | 0.34 (0.01–19.35) | 0.55 |
| Kampala | 4 (11.8) | 2.36 (1.42–3.91) | 0.002 | – | – |
| North | 2 (2.2) | 1.14 (0.42–3.10) | 0.74 | 1.36 (0.65–2.83) | 0.35 |
| West | 4 (2.3) | 0.65 (0.28–1.49) | 0.29 | 1.14 (0.00–356.92) | 0.96 |
| CD4 count at ART initiation | |||||
| ≥250 cells/mm3 | 2 (1.6) | 1 | 1 | ||
| <250 cells/mm3 | 11 (14.5) | 15.35 (1.08–218.28) | 0.05 | 30.6 (0.28–3386.92) | 0.13 |
| Initial ART regimen | 0.72 | ||||
| TDF+3TC+EFV/NVP | 24 (5.5) | 1 | |||
| ZDV+3TC+EFV/NVP | 3 (5.2) | 0.46 (0.07–3.25) | 0.42 | ||
| TDF+FTC+EFV | 1 (2.6) | 2.34 (0.32–16.96) | 0.38 | ||
Fourteen patients whose VL did not amplify and 10 with failed genotyping were not included in the analysis. CD4 counts of 15 patients were missing.
Age and gender were added to the multivariate model to control for confounding.
The ADR48 survey
Enrolment profile, and socio-demographic and clinical characteristics
A total of 1110 participants were enrolled; however, one site with 46 participants was excluded from the analysis following a protocol violation. This site had enrolled study participants with prior detectable VL which introduced selection bias, contrary to the consecutive enrolment process that was defined in the study protocol. Figure 1 summarizes the profile of the enrolled study participants. Among the 1064 participants included in this analysis, the median age was 44 years (IQR 37–51), median CD4 count at ART initiation was 214 cells/mm3 (IQR 126–310) and 34.7% of the participants were males. By the time of survey enrolment, 853 (80.2%) of the patients were maintained on their initial first-line regimen: TDF + 3TC + EFV (n = 192)/NVP (n = 95) or ZDV + 3TC + EFV (n = 84)/NVP (n = 482) and 211 (19.8%) had their regimen substituted, including all the 101 who had been initiated on stavudine (D4T). The median time on ART was 82 months (IQR 78.5–85.0) (Table 5).
Table 5.
Demographic, clinical and immunological characteristics of the 1064 ADR48 participants in Uganda
| Characteristics | Total |
|---|---|
| Gender, n (%) | |
| male | 369 (34.7) |
| female | 695 (65.3) |
| Age, years, median (IQR) | 44 (37-51) |
| Region, n (%) | |
| Central | 230 (21.6) |
| Eastern | 184 (17.3) |
| Kampala | 138 (13.0) |
| Northern | 230 (21.6) |
| Western | 282 (26.5) |
| CD4 count at ART initiation, cells/mm3, median (IQR) | 214 (126–310) |
| Viral load, n (%) | |
| <1000 copies/mL | 926 (87.0) |
| ≥1000 copies/mL | 138 (13.0) |
| VL among VFs, log10 copies/mL, median (IQR) | 3.7 (3.6–3.9) |
| Time on ART, months, median (IQR) | 82.0 (78.5–85.0) |
| Initial ART regimen, n (%) | |
| ZDV+3TC+EFV/NVP | 658 (61.8) |
| TDF+3TC+EFV/NVP | 305 (28.7) |
| D4T+3TC+EFV/NVP | 101 (9.5) |
HIV VL and DRMs at ADR48
The prevalence of VLS among all the 1064 patients was 87.9% (95% CI 85.0–90.9), shown in Figure 1 and Table 6, and the median VL among the 138 VFs was 3.7 log10 copies/mL (95% CI 3.6–3.9) (Table 5).
Table 6.
Weighted prevalencea of virological suppression and HIVDR among ADR48 participants
| Survey outcome | n/N | Percentage prevalence (95% CI) |
|---|---|---|
| VL suppression | ||
| all | 926/1064 | 87.9 (85.0–90.9) |
| male | 319/369 | 87.5 (83.0–92.2) |
| female | 607/695 | 88.2 (85.3–91.0) |
| age <44 years | 453/530 | 87.1 (83.7–90.5) |
| age ≥44 years | 473/534 | 88.7 (84.8–92.6) |
| HIVDR | ||
| any | 88/1064 | 7.6 (5.4–9.5) |
| NRTI | 84/1064 | 7.3 (5.2–9.5) |
| NNRTI | 86/1064 | 7.4 (5.1–9.7) |
| NRTI+NNRTI | 81/1064 | 7.0 (4.9–9.1) |
| HIVDR among VFs | ||
| any | 88/95 | 90.4 (73.6–96.8)b |
| NRTI | 84/95 | 86.8 (76.3–97.3) |
| NNRTI | 86/95 | 87.6 (76.4–98.8) |
| NRTI+NNRTI | 82/95 | 81.9 (71.2–92.6) |
All prevalences are weighted and accounted for the two-stage clustered survey design.
Log-transformed CI.
The overall prevalence of HIVDR was 7.6% (95% CI 5.4%–9.5%) as shown in Table 6. However, 88 of the 95 (90.4%; 95% CI 73.6%–96.8%) patients with VL ≥1000 copies/mL who were successfully genotyped had at least one DRM (Table 6). We observed that of the 88 patients that had HIVDR, 84 (86.8%; 95% CI 76.3%–97.3%) had NRTI mutations, 86 (87.6%; 95% CI 76.4%–98.8%) had NNRTI mutations, 82 (81.9%; 95% CI 71.2%–92.6%) had both NRTI and NNRTI mutations while none had PI mutations. Of the 88 patients with mutations, 82 (93.2%) had MI84V/I, 37 (42.0%) had K103N/S, 24 (27.3%) had Y181C and 46 (52.3%) had TAMs. Table S2 summarizes the mutations among the 88 participants.
Factors associated with VF and HIVDRMs at ADR48
Initiation on a zidovudine-based regimen compared with a tenofovir-based regimen was significantly associated with VF among patients receiving ART for at least 48 months (AOR 1.96, 95% CI 1.13–3.39, P = 0.02). There was a weak association between patients who had been on ART for at least 82 months compared with those who had been on ART for <82 months (AOR 1.77, 95% CI 0.94–3.33 P = 0.07) (Table 7).
Table 7.
Independent factors associated with virological failure among the 138 ADR48 participants in Uganda
| Univariate analysis |
Multivariate analysisa |
||||
|---|---|---|---|---|---|
| Characteristics | VL ≥1000 copies/mL, n (%) | crude OR (95% CI) | P value | adjusted OR (95% CI) | P value |
| Gender | |||||
| female | 88 (12.7) | 1 | 1 | ||
| male | 50 (13.6) | 1.07 (0.73–1.57) | 0.73 | 0.96 (0.67–1.34) | 0.79 |
| Age | |||||
| ≥44 years | 61 (11.4) | 1 | 1 | ||
| <44 years | 77 (14.5) | 1.16 (0.77–1.75) | 0.45 | 1.05 (0.68–1.62) | 0.80 |
| Region | 0.84 | ||||
| Central | 33 (14.4) | 1 | |||
| East | 26 (14.1) | 1.24 (0.49–3.15) | 0.63 | ||
| Kampala | 19 (13.8) | 1.25 (0.59–2.67) | 0.54 | ||
| North | 30 (13.0) | 1.07 (0.44–2.60) | 0.88 | ||
| West | 30 (10.6) | 0.87 (0.43–1.77) | 0.69 | ||
| CD4 count at ART initiationb | |||||
| <250 cells/mm3 | 76 (13.6) | 1 | |||
| ≥250 cells/mm3 | 43 (11.8) | 0.83 (0.50–1.34) | 0.50 | ||
| Time on ART | |||||
| <82 months | 55 (11.0) | 1 | 1 | ||
| ≥82 months | 81 (14.9) | 1.45 (0.89–2.36) | 0.12 | 1.77 (0.94–3.33) | 0.07 |
| Initial ART regimen | 0.37 | ||||
| TDF+3TC+EFV/NVP | 32 (10.5) | 1 | 1 | ||
| ZDV+3TC+EFV/NVP | 97 (14.7) | 1.45 (0.81–2.63) | 0.20 | 1.96 (1.13–3.39) | 0.02c |
| D4T+3TC+EFV/NVP | 9 (8.9) | 0.97 (0.48–1.96) | 0.91 | 1.70 (0.66–4.37) | 0.25 |
Age and gender were added to the multivariate model to control for confounding.
Nineteen patients had missing CD4 counts, and two patients had time on ART missing.
Statistically significant (P < 0.05).
Independent predictors for HIVDR among patients receiving ART for at least 48 months were: ART duration of at least 82 months compared with patients on ART for less than 82 months (AOR 1.92, 95% CI 1.03–3.59, P = 0.04) and initiating on a zidovudine-based regimen compared with a tenofovir-based regimen (AOR 3.16, 95% CI 1.34–7.46, P = 0.01) (Table 8).
Table 8.
Predictors of HIVDR in the 88 ADR48 participants receiving ART in Uganda
| Univariate analysis |
Multivariate analysisa |
||||
|---|---|---|---|---|---|
| Characteristics | HIVDRMs, n (%) | crude OR (95% CI) | P value | adjusted OR (95% CI) | P value |
| Gender | |||||
| male | 33 (6.5) | 1 | 1 | ||
| female | 55 (10.8) | 1.54 (0.62–1.54) | 0.91 | 0.94 (0.63–1.39) | 0.73 |
| Age | |||||
| ≥44 years | 57 (8.5) | 1 | 1 | ||
| <44 years | 31 (8.8) | 1.05 (0.66–1.68) | 0.82 | 1.42 (0.83–2.44) | 0.19 |
| Region | |||||
| Central | 22 (10.5) | 1 | |||
| East | 17 (9.6) | 1.21 (0.45–3.20) | 0.69 | ||
| Kampala | 12 (9.1) | 1.12 (0.44–2.85) | 0.79 | ||
| North | 17 (7.8) | 0.94 (0.33–2.71) | 0.90 | ||
| West | 20 (7.3) | 0.82 (0.37–1.85) | 0.62 | ||
| CD4 count at ART initiationb | |||||
| <250 cells/mm3 | 49 (9.1) | 1 | |||
| ≥250 cells/mm3 | 26 (7.5) | 0.79 (0.42–1.45) | 0.44 | ||
| Time on ART | |||||
| <82 months | 33 (6.9) | 1 | 1 | ||
| ≥82 months | 53 (10.2) | 1.58 (1.01–2.48) | 0.05 | 1.92 (1.03–3.59) | 0.04c |
| Initial ART regimen | |||||
| TDF+3TC+EFV/NVP | 16 (5.5) | 1 | 1 | ||
| ZDV+3TC+EFV/NVP | 69 (10.9) | 2.08 (0.87–4.99) | 0.10 | 3.16 (1.34–7.46) | 0.01c |
| D4T+3TC+EFV/NVP | 3 (3.1) | 0.85 (0.30–2.44) | 0.75 | 1.84 (0.46–7.57) | 0.34 |
Age and gender were added to the multivariate model to control for confounding.
A total of 43 patients with failed genotyping were not included in the analysis. CD4 counts of 13 patients were missing.
Statistically significant (P < 0.05).
Discussion
To our knowledge, this is the first report highlighting the predictors of VF, weighted prevalence and patterns of HIVDR among patients on first-line ART in Uganda using the WHO protocol.
The VLS rates from this survey were very similar to the findings of a descriptive cross-sectional study conducted in Uganda between 2014 and 2015 using routinely collected programme data from VL samples collected across the country that found an overall VF of 11%.22 In that survey, however, they included all patients on ART irrespective of age, regimen or duration on ART (for >6 months) and they defined VF as those with plasma VL ≥1000 copies/mL or DBS VL ≥5000 copies/mL, which could have made it less comparable to the present study. A much earlier survey in Uganda between 2012 and 2013 that implemented a WHO HIVDR survey protocol at three treatment centres found VLS at 85% and ADR at 9% among patients on first-line ART for 12 months and associated VF with high baseline VL and type of treatment facility.11 Although we did not find any factors associated with VF among the ADR12 patients, the ADR48 patients on ART for more than 82 months and who initiated on a zidovudine-based regimen compared with a tenofovir-based regimen were most likely to have VF. In contrast, under the Uganda national VL programme, VF was associated with young age, poor adherence and having active tuberculosis.22
In this study, although the VLS rates were high, we found that almost all participants that had VF and were genotyped had DRMs. The high HIVDR among those failing first-line therapy suggests that failure should be followed by an immediate switch since the recommended PI-based second-line or dolutegravir-based regimens currently have minimal resistance levels. The current recommendation of three intensive adherence counselling (IAC) sessions 1 month apart followed by repeat VL testing on the third IAC visit prior to treatment switch urgently needs to be reviewed especially since IAC would not be beneficial to the majority of VFs in this study. A recent report in Uganda has shown very low VLS rates (23%) following IAC among children and adolescents, of whom only 50% had completed the three IAC sessions23 and, among adults, close to 70% do not return for their scheduled second VL test according to the national VL programme (unpublished results). A study in Uganda and Zimbabwe among children receiving long-term ART without routine VL monitoring showed that among children who were retrospectively tested and found with viral rebound, none had re-suppressed, which demonstrated the limited utility of IAC among these children.24 From other selected cross-sectional surveys from South Africa among adults with VF on the first VL, very high levels of HIVDRMs were shown between 2010 and 2012 (86%)25 and in 2013 (89%).26 The immediate switching of unsuppressed individuals avoids keeping them on failing regimens with the potential to transmit HIVDR. These, along with our findings, should prompt an urgent review of the IAC approach.
The risk of VF and HIVDR at 48 months is almost comparable to that at 12 months, implying that adherence support and retention in care initiatives are beneficial. The first-line regimens used by study participants that developed the highest resistance are in the process of being phased out as the country optimizes regimens to shift to a dolutegravir (DTG)-based regimen. The recommendation by WHO for change of regimen to TDF + 3TC+DTG was due to the high pre-treatment drug resistance to NNRTIs, better tolerability, superior efficacy and higher genetic barrier of dolutegravir. There were earlier dolutegravir safety concerns reported among women of reproductive potential in Botswana showing that 0.9% (4/426) of babies whose mothers became pregnant while taking dolutegravir had a neural tube defect compared with 0.1% (14/11 173) of babies whose mothers were on other regimens.27,28 However, recent results have not shown a clear link of dolutegravir to neural tube defects29,30 and subsequently the WHO has updated the treatment guidelines that include the use of dolutegravir in children and adults including pregnant women for first- and second-line regimens.31 The WHO has further recommended increased surveillance to definitively confirm the effect of dolutegravir on neural tube defects and to provide information about the risks and benefits of dolutegravir for childbearing women in order to make informed choices.
Our study did have some limitations that were mainly technical and included the genotyping and VL testing failures that could possibly be attributed to the use of DBS samples. In resource-limited settings, DBS has been validated and recommended for HIVDR surveillance surveys due to its cost effectiveness and ease of transportation;16,32 however, it has some limitations compared with testing of plasma. The PCR efficiency of DBS is reduced when VLs are <5000 copies/mL, and the RNA integrity is further affected by sample quality due to poor blood collection techniques, and DBS preparation, transportation and storage. The use of DBS for VL quantification has been shown to present some false-positive results due to proviral DNA contamination at low VL copies,33 and this may lead to an overestimation of VF and underestimation of ADR.
The DBS samples used in this study were collected and transported to our lab using the routine National Sample and Results Transport Network.17 The overall genotyping success rate was 69.1%; however, among the samples that failed genotyping, 75% had a VL between 1000 and 5000 copies/mL while those with VL >5000 copies/mL had success rates of 87% (Table S3).
Results from this study that have demonstrated high VLS rates but very high HIVDR among patients with VF should be of concern with regard to onward transmission of HIVDR. The WHO has recommended roll-out of dolutegravir, a drug with a high genetic barrier to drug resistance, and this should be coupled with baseline surveys among patients initiating or switching to dolutegravir-based regimens to estimate the prevalence and profile of HIVDRMs to integrase inhibitors.
Conclusions
While VLS was generally good among individuals on first-line ART at 12 and 48 months, we found a high prevalence of ADR among the VFs before they underwent the recommended IAC. The three IAC sessions 1 month apart followed by repeat VL on the third IAC may be of limited benefit in improving VLS, and the delayed switching may increase the risk of HIV transmission and accumulation of DRMs, thus further compromising the effectiveness of the second-line regimens.
Supplementary Material
Acknowledgements
The authors acknowledge all the facility staff and study participants. We thank members of the Uganda HIV Drug Resistance Technical Working Group.
Members of the Uganda HIV Drug Resistance Technical Working Group
Pontiano Kaleebu, UVRI and MRC/UVRI & LSHTM Uganda Research Unit, Entebbe (Chair); Wilford Kirungi, AIDS Control Programme, Ministry of Health (MOH) (Vice Chair); Paula Munderi, MRC/UVRI & LSHTM Uganda Research Unit, Entebbe; Francis Ssali, Joint Clinical Research Centre (JCRC); Tom Lutalo, UVRI and Rakai Health Sciences Programme; Bernard Etukoit, The AIDS Support Organization; Grace Namayanja–CDC Uganda; Christine Watera–UVRI; Helen Byomire, National Drug Authority; Andrew Kambugu, Infectious Diseases Institute; Cissy Kityo, JCRC; Norah Namuwenge, MOH; and Elizabeth Namagala, MOH.
Contributor Information
The Uganda HIV Drug Resistance Technical Working Group:
Pontiano Kaleebu, Wilford Kirungi, Paula Munderi, Francis Ssali, Tom Lutalo, Bernard Etukoit, Grace Namayanja, Christine Watera, Helen Byomire, Andrew Kambugu, Cissy Kityo, Norah Namuwenge, and Elizabeth Namagala
Funding
This project has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the CDC under the terms of cooperative agreement #5U2GGH000785-02. Other support is from the UK MRC and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement.
Transparency declarations
None to declare.
Author contributions
Conceived and designed the study: P.K., J.A., C.W., D.S., W.K., M.K., N.N., G.N., A.N., E.R. and E.K.M. Collected data: J.A., C.W., D.S., G.S., I.S. and U.K. Conducted laboratory analysis: P.K., D.S., G.S., I.S., M.N., F.N., S.L., M.G.S. and J.F.S.G. Analysed the data: P.K., J.A., C.W., D.S., T.L. and U.K. Wrote the paper: D.S., J.A., P.K., C.W., G.N., A.N., T.L., G.N., E.R. and E.K.M. All authors reviewed, revised and approved the final paper.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the funding agencies.
References
- 1.UNAIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. 2017. http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf.
- 2.WHO. WHO global strategy for the surveillance and monitoring of HIV drug resistance. 2012. https://www.who.int/hiv/pub/drugresistance/drug_resistance_strategy/en/.
- 3.WHO. Surveillance of HIV drug resistance in adults initiating antiretroviral therapy. 2014. http://www.who.int/hiv/pub/drugresistance/pretreatment_drugresistance/en/.
- 4.WHO. Surveillance of HIV drug resistance in adults receiving ART. 2014. http://www.who.int/hiv/pub/drugresistance/acquired_drugresistance/en/.
- 5. Ndembi N, Lyagoba F, Nanteza B. et al. Transmitted antiretroviral drug resistance surveillance among newly HIV type 1-diagnosed women attending an antenatal clinic in Entebbe, Uganda. AIDS Res Hum Retroviruses 2008; 24: 889–95. [DOI] [PubMed] [Google Scholar]
- 6. Ssemwanga D, Ndembi N, Lyagoba F. et al. Transmitted antiretroviral drug resistance among drug-naive female sex workers with recent infection in Kampala, Uganda. Clin Infect Dis 2012; 54 Suppl 4: S339–42. [DOI] [PubMed] [Google Scholar]
- 7. Ssemwanga D, Kapaata A, Lyagoba F. et al. Low drug resistance levels among drug-naive individuals with recent HIV type 1 infection in a rural clinical cohort in southwestern Uganda. AIDS Res Hum Retroviruses 2012; 28: 1784–7. [DOI] [PubMed] [Google Scholar]
- 8. Price MA, Wallis CL, Lakhi S. et al. Transmitted HIV type 1 drug resistance among individuals with recent HIV infection in East and Southern Africa. AIDS Res Hum Retroviruses 2011; 27: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ndembi N, Hamers RL, Sigaloff KC. et al. Transmitted antiretroviral drug resistance among newly HIV-1 diagnosed young individuals in Kampala, Uganda. AIDS 2011; 25: 905–10. [DOI] [PubMed] [Google Scholar]
- 10. Hamers RL, Wallis CL, Kityo C. et al. HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect Dis 2011; 11: 750–9. [DOI] [PubMed] [Google Scholar]
- 11. Kaleebu P, Kirungi W, Watera C. et al. Virological response and antiretroviral drug resistance emerging during antiretroviral therapy at three treatment centers in Uganda. PLoS One 2015; 10: e0145536.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crawford KW, Wakabi S, Kibuuka H. et al. East meets west: a description of HIV-1 drug resistance mutation patterns of patients failing first line therapy in PEPFAR clinics from Uganda and Nigeria. AIDS Res Hum Retroviruses 2014; 30: 796–9. [DOI] [PubMed] [Google Scholar]
- 13. Gupta RK, Gregson J, Parkin N. et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis 2018; 18: 346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCluskey SM, Lee GQ, Kamelian K. et al. Increasing prevalence of HIV pretreatment drug resistance in women but not men in rural Uganda during 2005–2013. AIDS Patient Care STDS 2018; 32: 257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gruner N, Stambouli O, Ross RS.. Dried blood spots—preparing and processing for use in immunoassays and in molecular techniques. J Vis Exp 2015; doi: 10.3791/52619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parry CM, Parkin N, Diallo K. et al. Field study of dried blood spot specimens for HIV-1 drug resistance genotyping. J Clin Microbiol 2014; 52: 2868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kiyaga C, Sendagire H, Joseph E. et al. Uganda’s new national laboratory sample transport system: a successful model for improving access to diagnostic services for Early Infant HIV Diagnosis and other programs. PLoS One 2013; 8: e78609.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kiyaga C, Sendagire H, Joseph E. et al. Consolidating HIV testing in a public health laboratory for efficient and sustainable early infant diagnosis (EID) in Uganda. J Public Health Pol 2015; 36: 153–69. [DOI] [PubMed] [Google Scholar]
- 19. Woods CK, Brumme CJ, Liu TF. et al. Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. J Clin Microbiol 2012; 50: 1936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu TF, Shafer RW.. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis 2006; 42: 1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO, CDC, Global Fund. HIV drug resistance report. 2017. https://apps.who.int/iris/bitstream/handle/10665/255896/9789241512831-engpdf?sequence=1.
- 22. Bulage L, Ssewanyana I, Nankabirwa V. et al. Factors associated with virological non-suppression among HIV-positive patients on antiretroviral therapy in Uganda, August 2014–July 2015. BMC Infect Dis 2017; 17: 326.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nasuuna E, Kigozi J, Babirye L. et al. Low HIV viral suppression rates following the intensive adherence counseling (IAC) program for children and adolescents with viral failure in public health facilities in Uganda. BMC Public Health 2018; 18: 1048.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szubert AJ, Prendergast AJ, Spyer MJ. et al. Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: observational analyses within the randomised ARROW trial. PLoS Med 2017; 14: e1002432.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manasa J, Lessells RJ, Skingsley A. et al. High-levels of acquired drug resistance in adult patients failing first-line antiretroviral therapy in a rural HIV treatment programme in KwaZulu-Natal, South Africa. PLoS One 2013; 8: e72152.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hunt GM, Dokubo EK, Takuva S. et al. Rates of virological suppression and drug resistance in adult HIV-1-positive patients attending primary healthcare facilities in KwaZulu-Natal, South Africa. J Antimicrob Chemother 2017; 72: 3141–8. [DOI] [PubMed] [Google Scholar]
- 27.Editorial. Dolutegravir for HIV: a lesson in pregnancy safety research. Lancet 2018; 391: 2296. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Statement on DTG. 2018. https://www.who.int/medicines/publications/drugalerts/Statement_on_DTG_18May_2018final.pdf.
- 29. Dugdale CM, Ciaranello AL, Bekker LG. et al. Risks and benefits of dolutegravir- and efavirenz-based strategies for South African women with HIV of child-bearing potential: a modeling study. Ann Intern Med 2019; 170: 614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phillips AN, Venter F, Havlir D. et al. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. Lancet HIV 2019; 6: e116–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO. Update of recommendations on first- and second-line antiretroviral regimens. 2019. https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf?ua=1.
- 32. Bertagnolio S, Soto-Ramirez L, Pilon R. et al. HIV-1 drug resistance surveillance using dried whole blood spots. Antivir Ther 2007; 12: 107–13. [PubMed] [Google Scholar]
- 33. Monleau M, Aghokeng AF, Eymard-Duvernay S. et al. Field evaluation of dried blood spots for routine HIV-1 viral load and drug resistance monitoring in patients receiving antiretroviral therapy in Africa and Asia. J Clin Microbiol 2014; 52: 578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.