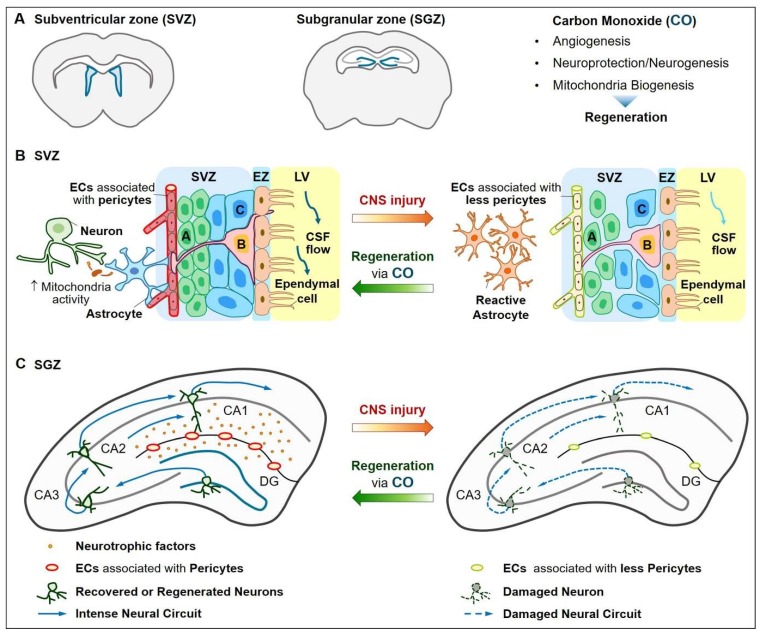
Figure 3.
Schematic figures showing possible regenerative roles of CO in the adult neurovascular communications. (A) Adult neurogenesis occurs in the subventricular zone (SVZ) and subgranular zone (SGZ), possibly supported by functional vessels. CO can induce angiogenesis, neuroprotection/neurogenesis and mitochondrial biogenesis, consequently facilitating regeneration. (B) Among adult SVZ neural stem cells (NSCs), radial glia-like NSCs (B cells) reside along the ependymal zone and extend a radial process to contact blood vessels, which are associated with pericytes. B cells generate transit-amplifying cells (C cells). B cells also extend a cilium through the ependymal zone (EZ) to contact the cerebrospinal fluid (CSF) in the lateral ventricle (LV). Neuroblasts (A cells) migrate and differentiate into olfactory bulb neurons. In adult NSC niches, astrocytes contribute to the cellular architecture by connecting endothelial cells and neurons as well as transferring mitochondria. Functional communication can be disrupted by CNS injury and CO may have the capacity to recover cell-cell communications after injury. CO may reduce the extent of reactive astrocytes. (C) Intense neural circuit formation by CO may be possible after CNS injury through coordinated neurovascular networks. Endothelial cells associated pericytes may form the blood-brain barrier and stimulate the release of various growth/neurotrophic factors from surrounding glial cells, which are mediated by therapeutic doses of CO. In CO exposed conditions after CNS injury, newly synthesized neurons in the dentate gyrus (DG) may connect new neural circuits through the cornus ammonis (CA)3-CA2-CA1 pathway.