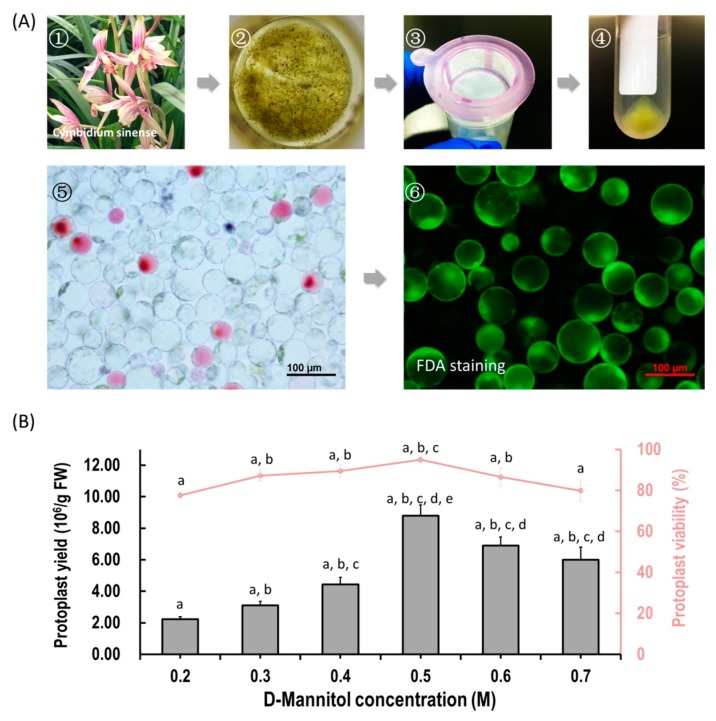
Figure 1.
Protoplast isolation from flower petals of Cymbidium orchids. (A) Schematic illustration of protoplast isolation from Cymbidium orchids. (1) Cymbidium flower petals were collected and cut into 0.5–1.0 mm strips using fresh surgical blades on sterile filter papers; (2) immediately, transfer strips into in a 100 mL flask containing enzymatic solution; (3) after 5 h incubation at 28 °C in dark, the solution containing released protoplasts were filter through a 150 μm nylon mesh into a 50 mL round-bottomed tube; (4) following centrifuge at 200 rpm for 2 min, the protoplast pellet were re-suspended with W5 solution; (5) check the morphology and yield of protoplasts under a microscope and (6) measure the protoplast viability using the FDA staining method. (B) Optimization of Cymbidium protoplast isolation conditions. A series of D-mannitol concentration in the enzyme solutions (0.2, 0.3, 0.4, 0.5, 0.6 and 0.7 M) were tested for different tissues to ensure that released protoplasts do not rupture or collapse during enzyme digestion; data presented as means of three biological replicates with error bars indicating standard deviations (SD), and different letters (a–e) among treatments indicate statistically significant differences at p < 0.05 based on the Tukey–Kramer test.