Figure 8.
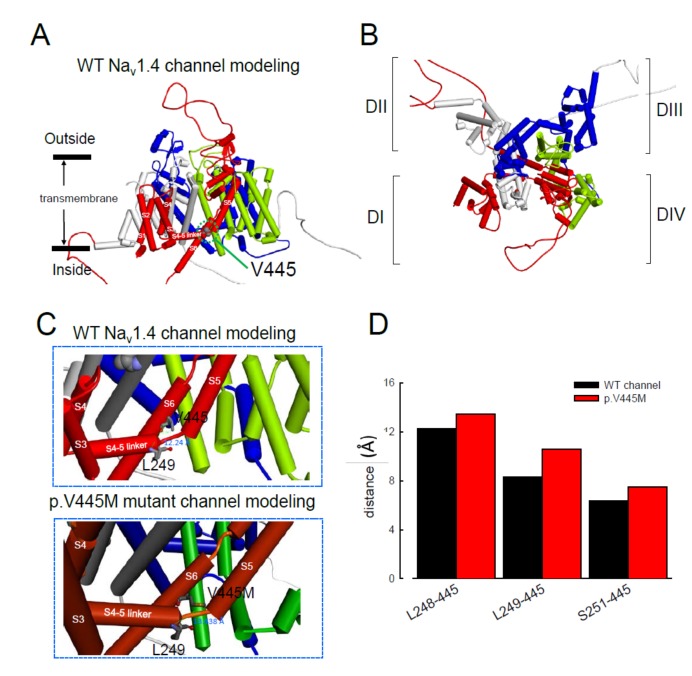
Homology modeling of the WT and mutant human Nav1.4 channels. (A) Side view of the homology model shows the transmembrane helixes of the four domains. The four domains I, II, III and IV are colored red, white, blue and green. The side chain of V445 in the S6 linker of Domain I (DI) is indicated in the CPK model. (B) A closer view of the WT Nav1.4 channel from the external side of the pore. (C) A diagram of Domain I of the homology model of the WT Nav1.4 and p.V445M mutant channels is shown in the schematic presentation. The side chains of V445 in the S6 segment and L248, L249 and S251 in the S4–5 linker of Domain I are shown with sticks of various colors. A closer view of the area is shown in the panel, demonstrating inter-residue distances of ~8.3196, ~12.24 and ~6.348 Å between V445 and L248, V445M and L249 and V445 and S251, respectively, in the WT Nav1.4 channel, while the distances between p.V445M and L248, p.V445M and S249 and p.V445M and S251 are ~7.54, ~13.438 and ~10.551 Å, respectively, in the p.V445M mutant channel. (D) The relative distances between the residue V445 and L248, L249 and S251 in the homology modeling is shown. Note the conspicuously decreased distances in all cases in the WT channel compared with those in the p.V445M mutant channel.