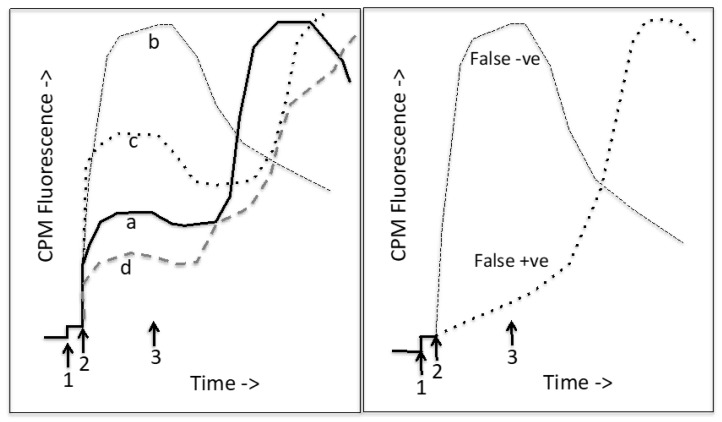
Figure 6.
Thermostability assay. CPM labeling experiments: CPM is added to the cuvette (1) at low temperature (e.g., 10 °C) and then the protein is added (2). After a period of labeling of surface exposed Cys residues, the cuvette is heated (starting at 3). Initially the CPM fluorescence decreases due to thermal quenching, but then as the protein unfolds, the CPM fluorescence rises, reaching a maximum after the protein is completely unfolded. After this, thermal quenching of the CPM fluorescence is again observed. Left panel shows typical CPM unfolding profiles for different membrane proteins. Trace a (the solid line) is for a membrane protein such as CFTR with about 40% of its 18 Cys residues exposed to the aqueous medium. It shows a single cooperative unfolding transition. Trace b (black, dashed) is for an unusual (perhaps very small) membrane protein with no buried Cys residues. Trace c (black, dotted) is for a very thermophilic membrane protein but with mostly exposed Cys residues. Trace d is for a membrane protein with few exposed Cys residues in the native state. It has multiple domains that show separate unfolding transitions. The right panel illustrates potential false results when examining the effects of drugs. A false negative drug (false destabilizer) will have a CPM-reactive group that reacts rapidly with CPM at low temperature. A false positive drug (apparent corrector) will have a CPM-reactive group that labels slowly at low temperature and then the rate of reaction speeds up upon heating.