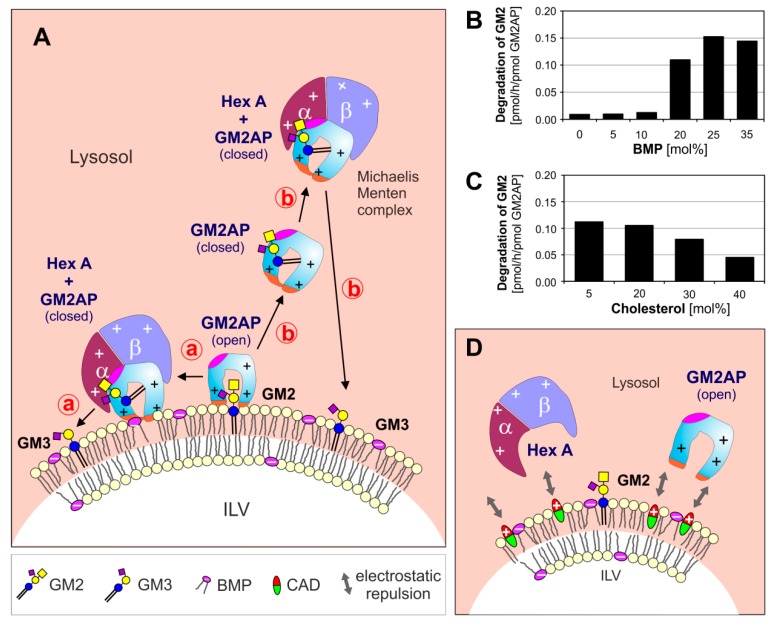
Figure 1.
(A) Model for enzymatic digestion of membrane bound GM2 by Hex A, assisted by GM2AP at the surface of ILV at low, lysosomal pH-values. The open and empty GM2AP conformation binds to the membrane, e.g., by affinity to its lipid ligand and charge dependent interaction of the cationic protein (+, positively charged) with negatively charged membrane lipids (−, negatively charged) like BMP. Thereafter, the activator can interact with the ceramide portion of the GM2-ligand, which can move inside the hydrophobic cavity of the GM2AP, exposing the glycan chain of the GM2 to the water-soluble Hex A for hydrolysis. At this point, the conformation of the lipid-loaded activator may change to the closed one, thus the complex becomes more water soluble and can either stay at the surface of the membrane (pathway ⓐ) or leave the membrane (pathway ⓑ). (B, C) The GM2 hydrolysis is affected by membrane lipids: (B) anionic lipids e.g., BMP stimulate and (C) cholesterol inhibits GM2 degradation [38]. (D) CADs reaching the lysosome behave like cationic amphiphilic lipids, insert into the membrane surface of the intralysosomal luminal vesicles (ILVs) and start to compensate their negative surface charge. This results in a decreasing electrostatic attraction between proteins and ILVs, and an increasing repulsion between positively charged lysosomal proteins and the CAD-containing ILV-membrane. BMP: bis(monoacylglycero)phosphate, CADs: cationic amphiphilic drugs, Chol: cholesterol, GM2AP: GM2 activator protein, Hex A: β-hexosaminidase A, ILV: intralysosomal luminal vesicles.