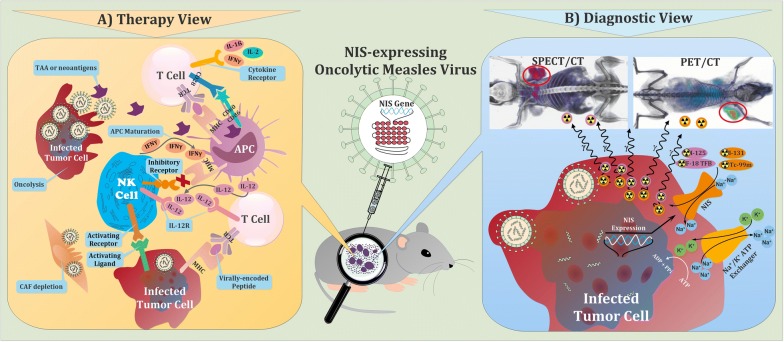
Fig. 1.
Schematic illustration of the multimodality role of virotheranostics in treatment and tracking of tumor cells. a Mechanisms by which oncolytic viruses (OVs) stimulate antitumor immunity. Virus-based immunostimulatory cytokine and chemokine expression can recruit and activate T cells, antigen-presenting cells (APCs), and natural killer (NK) cells, and subsequently, improve the therapeutic activity of OVs. Regardless of oncolysis, OVs stimulate innate immune receptors on professional antigen-presenting cells (APCs) such as dendritic cells and prime antitumor T cells. OVs are able to counteract immune suppression through several mechanisms, including stimulation of pro-inflammatory cytokines and TAAs production and also depletion of immunosuppressive cell types such as cancer-associated fibroblasts (CAFs) within the tumor microenvironment. OVs can also improve recognition of tumor cells by the immune system through upregulation of pathways involved in antigen processing and presentation, including increased major histocompatibility complex (MHC) class I and MHC class II expression on APCs and tumor cells. Subsequently, CD28 signaling will result in the activation of the CD8 + T cell. These activated CD8 + T cells will differentiate into effector T cells that can recognize the MHC class I-peptide complex on virally infected cells. Binding of the TCR to the MHC class I-peptide complex leads to activation of the CD8 + T cell and the release of cytokines. Moreover, NK cells play a pivotal role in detection and killing tumor cells, recruiting other immune cells, mediating T cell activation, and expanding and releasing chemokines and cytokines. The activation of NK cells depends upon the presence of local cytokines such as IL-12. Regulation of NK cells is achieved through binding of inhibitory cell surface receptors such as the killer-cell immunoglobulin receptors (KIRs) (not shown), which bind to different human leukocyte antigen (HLA) complexes on tumor cells. Upon some circumstances of cellular stress like viral infection, associated ligands for activating receptors are often upregulated and MHC class I expression may be downregulated. The upregulation of activating ligands and downregulation of MHC class I produce a signal for NK cells to become activated and play their effector functions. b Visualization of tumor cells through NIS-mediated cell imaging. Following transduction with the viral vector carrying the NIS gene, cancer cells are capable of transporting radioisotopes for imaging purposes. Tissue-specific promoter enables the NIS gene to express specifically in corresponding cancer cells, providing a promising strategy of cancer-targeting therapy and imaging. The cells can be imaged by radionuclide-based molecular imaging techniques using gamma-ray or positron-emitting radiotracers. Expression of sodium iodide symporter (NIS) reporter gene leads to the insertion of sodium iodide symporters into the cell membrane, where they import many reporter probes like TcO4−, ReO4−, and At−, along with sodium ion (Na +), into the cytosol. Imaging is performed with SPECT or PET and the results are like example pictures at top of panel B, in which tumor area has been determined by red circle