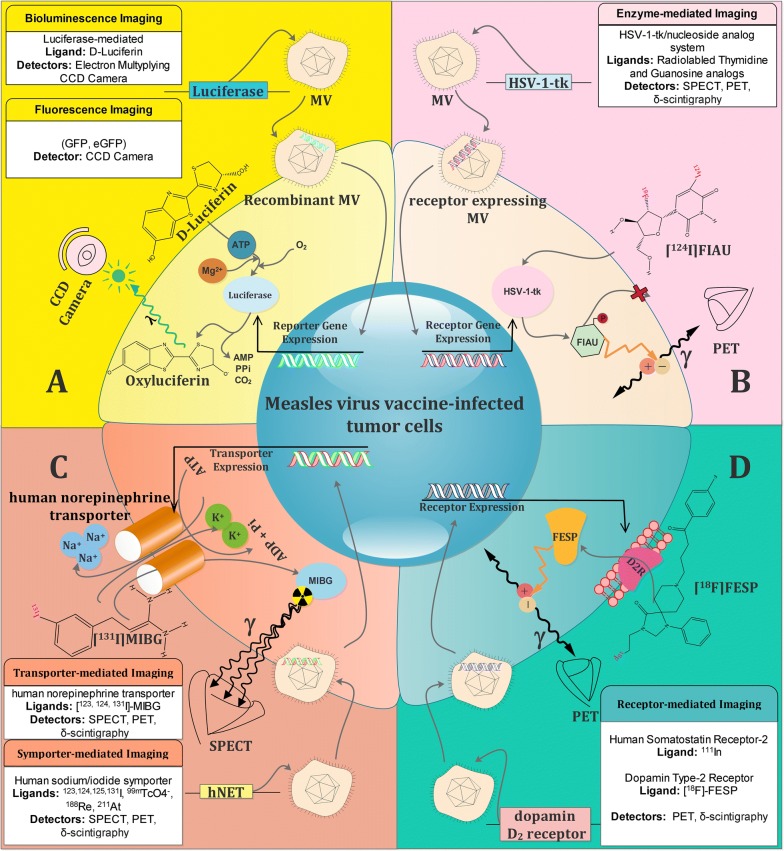
Fig. 2.
Schematic illustration of tumor cell imaging using noninvasive visualization systems by means of measles viruses (MVs) to encode reporter transgenes based on recombinant technology. a Optical imaging. Transcription and translation of the firefly luciferase (FLUC) gene lead to the accumulation of the firefly luciferase enzyme, which subsequently catalyzes a photochemical reaction in the presence of D-luciferin. This reaction yields low levels of fluorescence photons that can be detected and quantified by a charge-coupled device camera (CCD). b Enzyme-mediated imaging. Inside the transfected cells, HSV-1-tk is transcribed and translated to produce the HSV-1-tk enzyme. [124I]-FIAU is a labeled nucleoside analog substrate for HSV-1-tk. In the presence of HSV-1-tk, the radiolabeled probe is phosphorylated and trapped within the cell. Radioactive decay of 124I isotopes can be detected with PET. The magnitude of [124I]-FIAU signal reflects the activity of HSV-1-tk enzyme and thus HSV-1-tk gene expression. c Transporter/Symporter-mediated imaging. Tumor cells would acquire the function of iodine uptake with NIS gene transduction by viral vector delivery. NIS transports 2 sodium ions and 1 iodide ion into the cytoplasm together. The electrochemical sodium gradient generated by the Na+/K+ ATPase pump provides energy for this transfer. Gamma radiation of radiolabeled ligands such as [131I]-MIBG provide enough radiation for PET imaging. d Receptor-mediated imaging. 3-(2′-[18F]fluoroethyl)spiperone (FESP) is a gamma-emitting radiolabeled reporter probe that interacts with the dopamine 2 receptor (D2R) to result in probe trapping on or in cells expressing the D2R gene. Viral delivery of the D2R gene into the infected tumor cells armors them with a monitoring system, which can be visualized by PET