Abstract
Our previous work identified isoxazole-based chalcones and their dihydropyrazole derivatives as two important five-membered heterocycles having antitubercular activity. Hence, in the present study, we biologically evaluated 30 compounds, including 15 isoxazole ring-containing chalcones (17–31) and 15 dihydropyrazoles (32–46) derived from these chalcones for their antimicrobial, antioxidant, and anticancer activities. Chalcones exhibited superior antibacterial and antioxidant activities compared to dihydropyrazoles. Among the chalcones, compound 28 showed potent antibacterial (MIC = 1 µg/mL) and antioxidant activities (IC50 = 5 ± 1 µg/mL). Dihydropyrazoles, on the contrary, demonstrated remarkable antifungal and anticancer activities. Compound 46 (IC50 = 2 ± 1 µg/mL) showed excellent antifungal activity whereas two other dihydropyrazoles 45 (IC50 = 2 ± 1 µg/mL) and 39 (IC50 = 4 ± 1 µg/mL) exhibited potential anticancer activity. The compounds were also tested for their toxicity on normal human cell lines (LO2) and were found to be nontoxic. The active compounds that have emerged out of this study are potential lead molecules for the development of novel drugs against infectious diseases, oxidative stress, and cancer.
Keywords: isoxazole, chalcones, dihydropyrazole, antibacterial activity, antifungal activity, anticancer activity
1. Introduction
Heterocyclic compounds are a class of cyclic organic compounds containing heteroatoms, like nitrogen, sulfur, oxygen, etc., along with the carbon framework. The heterocyclic compounds possess diverse pharmacological activities and are employed in the treatment of a variety of diseases. Most of the therapeutic agents employed in the present-day therapy contain heterocyclic ring as the major structural component. Among these compounds, nitrogen-containing heterocyclic rings are distinctive not only because of their ease of synthesis but also due to their widespread distribution and biological profiles. Chalcones are open chain flavonoids containing the reactive propenone linker connected to two aryl rings. A literature survey revealed that chalcones and nitrogen-containing heterocycles, i.e., isoxazoles and dihydropyrazoles, possess a broad spectrum of biological activities, like antimicrobial, antioxidant, anticancer, antimalarial, antidepressant, antihistaminic, antitubercular, and anti-inflammatory [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
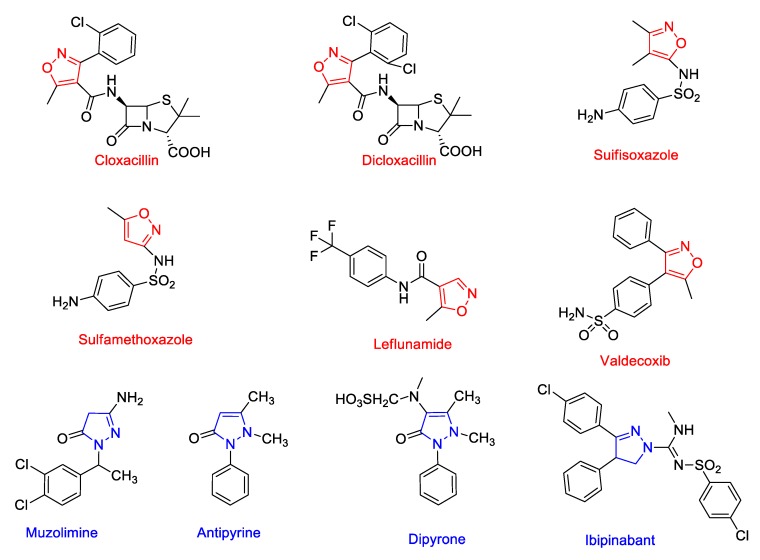
Isoxazole, a five-membered heterocyclic ring, is present in therapeutic drugs, including the β-lactam antibiotics, cloxacillin and dicloxacillin, sulfisoxazole and sulfamethoxazole (antibacterials), valdecoxib (selective COX-II inhibitor), and leflunomide, an immunosuppressive disease-modifying antirheumatic drug [DMARD]. The completely reduced form of isoxazole, i.e., isoxazoline and isoxazolidine scaffolds, are seen in the antifungal agent drazoxolon and antitubercular antibiotic cycloserine, respectively. Dihydropyrazole is another interesting heterocyclic compound with two nitrogen atoms present in the adjacent positions of a five-membered ring. This ring can be conveniently synthesized from α, β-unsaturated carbonyl compounds by reacting with different kinds of hydrazines. The dihydropyrazole or its oxo derivative scaffold constitutes the part of drugs, including Muzolimine (Diuretic); Antipyrine, Aminopyrine, Ramifenazone, and Dipyrone (Analgesics); and Ibipinabant (anti-obesity cannabinoid receptor type 1 ((CB1) antagonist) (Figure 1).
Figure 1.
Structures of some clinically useful drugs containing isoxazole and dihydropyrazole rings.
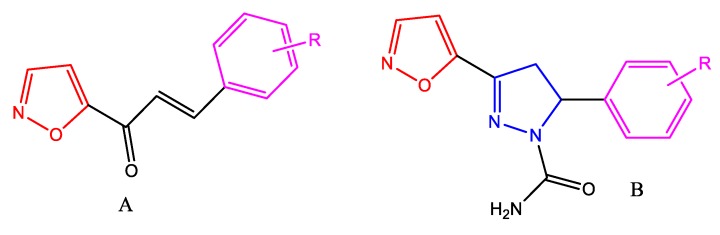
Chalcones, a privileged structure in medicinal chemistry [6], are a type of open chain flavonoids bearing two aryl rings connected through a three-carbon spacer, the propenone linkage. The reactive α, β-unsaturated propenone fragment is not only responsible for the bioactivities but is also useful for the conversion of chalcones to different classes of heterocyclic compounds. There is a difference in the intensity of the bioactivities shown by chalcones due to variation in the nature and type of aryl rings. A literature survey on chalcones revealed that compounds containing heteroaromatic rings in their aryl portion showed excellent biological profiles [7,8]. We have previously reported heterocyclic ring-appended chalcones exhibiting antitubercular, antifungal, and cytotoxic activities [9,10,11,12,13]. Based on a literature survey [1,2,3,4,5,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] and our previously conducted studies [9,10,11,12,13], we herein report a continuation of that work, with a goal of further evaluating isoxazole-containing chalcones and its dihydropyrazole derivatives (privileged heterocyclic compounds) (Figure 2) for their antibacterial, antifungal, antioxidant, and anticancer activities. The results of these efforts and the structure–activity relationship for the series of isoxazole chalcones and dihydropyrazole derivatives are discussed here.
Figure 2.
Previously designed isoxazole chalcone and dihydropyrazole derivatives.
2. Results and Discussion
2.1. Chemistry
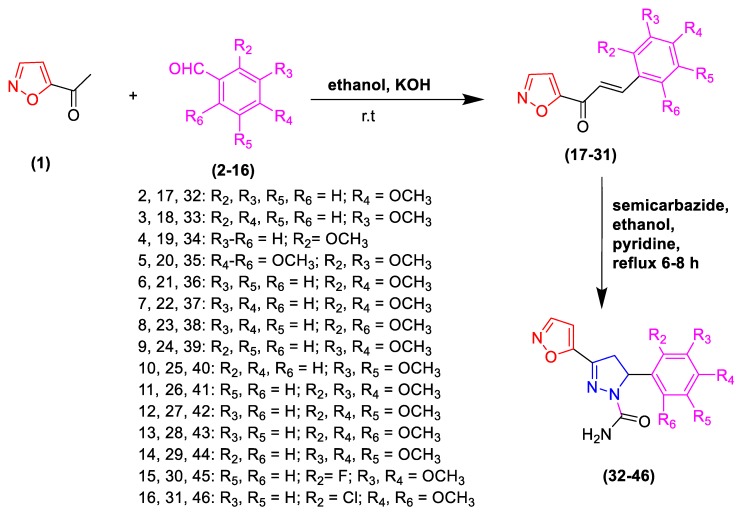
The synthesis and characterization of compounds 17–31 and 32–46 has been published previously [9,11]. Chalcones (17–31) were prepared by Claisen–Schmidt condensation reaction (Scheme 1) in the presence of aqueous potassium hydroxide as a catalyst. The 1-(isoxazole-5-yl)ethanone (1) was condensed with substituted benzaldehyde (2–16) under basis conditions at room temperature for 24 h. The reaction was stopped by transferring the mixture on ice. The crude precipitate was recrystallized using chloroform to afford chalcones 17–31. The target dihydropyrazoles (32–46) were obtained by treating the synthesized chalcones with semicarbazide in a catalytic amount of pyridine and purified using silica gel column chromatography.
Scheme 1.
Synthesis of isoxazolylchalcones (17–31) and dihydropyrazoles (32–46).
2.2. Biological Studies
2.2.1. Antibacterial and Antifungal Activities
Previously published studies suggested that chalcones/heteroarylchalcones and compounds having dihydropyrazoles exhibited antimicrobial, antioxidant, and cytotoxic activity [1,2,3,4,5,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. Hence, we decided to screen our compounds for antibacterial, antifungal, antioxidant, and anticancer (prostrate cell line) activities. The target chalcones (17–31) and pyrazolines (32–46) were tested for their antimicrobial activities by serial tube dilution method against two types of bacterial and fungal strains (Table 1 and Table 2). The bacterial strains used for the testing, including Gram-positive Staphylococcus aureus and Gram-negative Pseudomonas aeruginosa, whereas the fungal strains employed were Aspergillus niger and Candida tropicalis.
Table 1.
Results of the antibacterial and antifungal activities of chalcones (MIC µg/mL; 17–31).
| Compound | S aureus | P aeruginosa | A niger | C tropicalis |
|---|---|---|---|---|
| 17 | 4 | 8 | 8 | 8 |
| 18 | 128 | 128 | 256 | 256 |
| 19 | 4 | 4 | 8 | 16 |
| 20 | 32 | 64 | 64 | 32 |
| 21 | 2 | 4 | 8 | 16 |
| 22 | 32 | 32 | 64 | 64 |
| 23 | 4 | 4 | 4 | 4 |
| 24 | 64 | 32 | 128 | 64 |
| 25 | 256 | 256 | 256 | 512 |
| 26 | 64 | 64 | 128 | 128 |
| 27 | 16 | 16 | 32 | 32 |
| 28 | 1 | 1 | 2 | 2 |
| 29 | 64 | 128 | 128 | 256 |
| 30 | 4 | 4 | 16 | 16 |
| 31 | 2 | 2 | 4 | 4 |
| Ciprofloxacin | 2 | 2 | --- | --- |
| Fluconazole | --- | --- | 1 | 1 |
Table 2.
Results of the antibacterial and antifungal activity of dihydropyrazoles (MIC µg/mL; 32–46).
| Compound | S aureus | P aeruginosa | A niger | C tropicalis |
|---|---|---|---|---|
| 32 | 16 | 16 | 4 | 4 |
| 33 | 128 | 128 | 64 | 64 |
| 34 | 16 | 16 | 8 | 8 |
| 35 | 64 | 64 | 16 | 16 |
| 36 | 8 | 8 | 4 | 8 |
| 37 | 64 | 32 | 32 | 32 |
| 38 | 32 | 16 | 2 | 2 |
| 39 | 128 | 64 | 32 | 32 |
| 40 | 256 | 256 | 64 | 64 |
| 41 | 64 | 128 | 64 | 64 |
| 42 | 32 | 16 | 16 | 16 |
| 43 | 4 | 4 | 2 | 2 |
| 44 | 64 | 256 | 16 | 16 |
| 45 | 8 | 8 | 0.5 | 1 |
| 46 | 8 | 8 | 0.5 | 0.5 |
| Ciprofloxacin | 2 | 2 | --- | --- |
| Fluconazole | --- | --- | 1 | 1 |
Chalcones exhibited excellent antibacterial potency whereas the dihydropyrazoles showed tremendous antifungal activity compared to the standard drugs ciprofloxacin and fluconazole. In chalcones and dihydropyrazoles, the nature and position of the substituents on the phenyl ring played a crucial role for the antimicrobial activity. Compounds 17–29 had an electron-donating group (-OCH3) substituted at positions 2–6 on the aromatic ring and compounds 30 and 31 had electron-withdrawing groups (F, Cl) substituted at position 2 and electron-donating groups at position 3–6 on the aromatic ring. Compounds 17–19 represent monosubstituted chalcone derivative; 20–25 represent disubstituted chalcone derivative and compounds 26–31 represent trisubstituted chalcone derivative. In the monosubstituted chalcone series 17–19, the methoxy group at position 2 and 4 showed better antibacterial and antifungal activity than at position 3 but lower than the standard drugs. Among the disubstituted chalcone series 20–25, substitution at positions 2 and 6 showed better antibacterial activity than at positions 2,3 (20); 2,4 (21); 2,5 (22); 3,4 (24); and 3,5 (25). However, these compounds had lower activity than the standard drugs. In the trisubstituted series, 28 containing a 2,4,6-trimethoxyphenyl ring was the most potent (MIC 1 µg/mL) among the chalcone series and its antibacterial activity was found to be greater than ciprofloxacin. In contrast, the antifungal potency of 28 was found to be 2 µg/mL, which was less compared to fluconazole (1 µg/mL). On comparing compounds 17, 19, 23, and 28, it can be observed that the methoxy substituent is favorable to be substituted at positions 2 or 4 (17 and 19), 2 and 6 (23), or 2, 4 and 6 (28). Compound 31 containing 2-chloro-4,6-dimethoxyphenyl was next in potency to 28 with respect to antibacterial and antifungal activities. On comparing compounds 28 and 31, it can be seen that substituting the electron-donating group instead of the electron-withdrawing group at position 2 was favorable for their antibacterial and antifungal activities. Most of the chalcones demonstrated moderate antibacterial and antifungal activity, with the minimum inhibitory concentration (MIC) ranging from 4–64 µg/mL. Some of the compounds viz., 18, 25, 26, and 29 showed poor activity, with the MIC ranging between 64 and 256 µg/mL. It can be concluded from the structure–activity relationship studies that the presence of additional methoxy groups at the ortho and para positions (2, 4, and 6) was critical for the antimicrobial activity whereas substitution of methoxy groups in meta position (3) was deleterious for the antimicrobial and antifungal potency.
Dihydropyrazoles 32–46 exhibited better antifungal than antibacterial activity. As observed in the previous chalcone series, the nature and position of the substituents on the phenyl ring played a crucial role for the antifungal activity. Compounds 32–44 had an electron-donating group (-OCH3) substituted at positions 2-6 on the aromatic ring and compounds 45 and 46 had electron-withdrawing groups (F, Cl) substituted at positions 2 and electron-donating groups at positions 3-6 on the aromatic ring. Compounds 32–34, 35–40, and 41–46 represent monosubstituted, disubstituted, and trisubstituted dihydropyrazole derivatives. In the monosubstituted dihydropyrazole series, the substitution at position 4 (32) was found to be better than position 2 (34) for the antifungal activity (MIC 4 vs. 8 µg/mL). Methoxy substitution at position 3 (33) resulted in poor antibacterial and modest antifungal activity. Among the disubstituted dihydropyrazole series 35–40, substitution at positions 2 and 6 (38) showed better antifungal activity (MIC 2 µg/mL) than at positions 2,3 (35); 2,4 (36); 2,5 (37); 3,4 (39); and 3,5 (40). However, compound 38 had lower antifungal activity than the standard drug fluconazole (MIC 1 µm/mL). In the trisubstituted series, compound 43 (2 µg/mL) did not show any marked increase in antifungal activity compared to compound 38 (2 µg/mL). Compound 43, however, had an 8- and 4-fold improvement in the antibacterial activity compared to compound 38, which indicated that the methoxy group at position 4 benefitted the antibacterial over antifungal activity. The trisubstituted series also had the presence of electron-donating and -withdrawing groups (45 and 46). Structure activity relationship studies (SAR) studies suggested that halogen substituents on the phenyl ring (45 and 46) exhibited brilliant antifungal activity compared to fluconazole and improved their antibacterial activity by 8-fold for 45 (MIC 8µg/mL) vs. 41 (MIC 64 µg/mL) but was lowered for 46 (MIC 8 µg/mL) vs. 43 (MIC 4 µg/mL). The antifungal activity of compounds 45 and 46 was found to be the best in this series and better than the standard drug fluconazole. In general, the antibacterial potency of dihydropyrazoles was found to be less than that of chalcones (43 MIC 4 µg/mL vs. 28 1 µg/mL).
2.2.2. Antioxidant Activity
The antioxidant activity of all 30 compounds was assessed using 1,1-diphenyl-2-picrylhydrazine (DPPH) free radical scavenging assay and the results are the average of three independent experiments (Table 3). Gallic acid was employed as a positive control. Both isoxazole-based chalcones and dihydropyrazole derivatives exhibited significant antioxidant activity. In the chalcone monosubstituted series (17, 18, and 19), substituting at positions 2 or 4 or 6 on the phenyl ring did not improve the antioxidant activity. In the disubstituted series (20–25), compound 25 with substitutions at position 3 and 5 on the phenyl ring gave the highest activity of 9 µg/mL. In the trisubstituted series (26–31), substitutions at positions 2, 4, and 6 resulted in the highest IC50 of 5 µg/mL among all the series. Replacement of the methoxy group (26 and 28) with either F (30) or Cl (31) resulted in a drop in the antioxidant activity, which suggested that electron-donating groups are favored at positions 2 on the phenyl ring. A similar phenomenon was observed in the dihydropyrazole series (32–46). Compound 43 was found to have an IC50 of 6 µg/mL and was the most potent in this series. However, its activity was found to be less than the standard gallic acid (IC50 = 5 µg/mL) It can be concluded from the chalcone and dihydropyrazole data that the antioxidant activity was found to be improved on the substitution of the electron-donating group (-OCH3) on the phenyl ring at positions 2, 4, and 6. In the chalcone series, compound 28 containing three methoxy groups at positions 2, 4, and 6 was the most potent of the 30 compounds and its activity was equal to that of the standard, having an IC50 5 µg/mL.
Table 3.
Comparison of the DPPH assay results of chalcones (17–31) and dihydropyrazoles (32–46) (IC50 values in µg/mL).
| Compound | IC50 | Compound | IC50 |
|---|---|---|---|
| 17 | 33 ± 1 | 32 | 44 ± 1 |
| 18 | 38 ± 2 | 33 | 51 ± 2 |
| 19 | 32 ± 2 | 34 | 38 ± 2 |
| 20 | 24 ± 1 | 35 | 32 ± 1 |
| 21 | 18 ±2 | 36 | 25 ± 2 |
| 22 | 26 ±2 | 37 | 39 ± 2 |
| 23 | 16 ±1 | 38 | 28 ± 1 |
| 24 | 29 ±1 | 39 | 49 ± 1 |
| 25 | 9 ± 1 | 40 | 12 ± 1 |
| 26 | 6 ± 1 | 41 | 8 ± 1 |
| 27 | 7 ± 2 | 42 | 10 ± 2 |
| 28 | 5 ± 1 | 43 | 6 ± 1 |
| 29 | 8 ± 2 | 44 | 12 ± 2 |
| 30 | 45 ± 2 | 45 | 55 ± 2 |
| 31 | 48 ± 1 | 46 | 62 ± 1 |
| Gallic acid 5 ± 1 | |||
2.2.3. Anticancer Activity
All the 30 compounds were evaluated for their anticancer potency against the prostate cancer cell line, DU-145, employing colorimetric tetrazolium dye (MTT) assay. In the chalcone monosubstituted series 17–19, substituting at positions 2, 4, and 6 did not improve the anticancer activity. Among the disubstituted series 20–25, substitution at position 3 and 4 (compound 24) produced the best activity but was lower than the standard. Among the trisubstituted series 26–31, having an electron-withdrawing (F) and -donating groups (-OCH3) at position 2, 3, and 4 (compound 30) resulted in the highest potency among the chalcone series. This suggests that electron-withdrawing and -donating groups on the aromatic ring were required for improving the potency among the chalcone series. Compound 30, the most potent chalcone, was equipotent to the standard Docetaxel at IC50 5 ± 1 µg/mL. Similar phenomena can be seen among the dihydropyrazole series. In the monosubstituted series 32–34, substituting position 2, 4, or 6 did not yield any improvement in the activity. In the disubstituted series 35–40, compound 39 having electron-donating substitution at 3 and 4 was found to be the most potent, with IC50 4 ± 2 µg/mL. In the trisubstituted series 41–46, compound 45 was found to be the most potent having fluoro at position 2 and –OCH3 at positions 3 and 4, having an IC50 of 2 ± 1 µg/mL. It can be seen that the dihydropyrazole derivatives exhibited superior activity than the chalcones 39 vs. 24 and 45 vs. 30 (Table 4). The SAR features indicated that heterocyclic pyrazoline ring was more essential than the propenone motif found in chalcones. The activity of compounds 39 and 45 was greater than the standard, Docetaxel (MIC = 5 µg/mL). Chalcone 30 and dihydropyrazole 44 exhibited equipotent activity as that of the standard. The compounds 24, 29, 41, and 26 were next in activity, with IC50 values of 6, 8, 9, and 10 µg/mL, respectively. All the other compounds exhibited modest anticancer activity, with IC50 values ranging from 11–32 µg/mL. The compounds were also tested against the human normal cells (L02) and were found to be nontoxic to these cells.
Table 4.
Comparison of the MTT assay anticancer results of chalcones (17–31) and dihydropyrazoles (32–46) (IC50 values in µg/mL).
| Compound | DU-145 | Human Normal Cells (L02) | Compound | DU-145 | Human Normal Cells (L02) |
|---|---|---|---|---|---|
| 17 | 32 ± 2 | >40 | 32 | 28 ± 1 | >40 |
| 18 | 20 ± 1 | >40 | 33 | 18 ± 2 | >40 |
| 19 | 36 ± 1 | >40 | 34 | 32 ± 2 | >40 |
| 20 | 22 ± 1 | >40 | 35 | 16 ± 2 | >40 |
| 21 | 29 ± 2 | >40 | 36 | 26 ± 2 | >40 |
| 22 | 38 ± 1 | >40 | 37 | 31 ± 2 | >40 |
| 23 | 33 ± 2 | >40 | 38 | 20 ± 2 | >40 |
| 24 | 6 ± 2 | >40 | 39 | 4 ± 2 | >40 |
| 25 | 12 ± 1 | >40 | 40 | 8 ± 2 | >40 |
| 26 | 10 ± 2 | >40 | 41 | 9 ± 1 | >40 |
| 27 | 26 ± 3 | >40 | 42 | 18 ±2 | >40 |
| 28 | 30 ± 2 | >40 | 43 | 21 ± 2 | >40 |
| 29 | 8 ± 2 | >40 | 44 | 5 ± 2 | >40 |
| 30 | 5 ± 2 | >40 | 45 | 2 ± 1 | >40 |
| 31 | 16 ±2 | >40 | 46 | 12 ± 2 | >40 |
| Docetaxel 5 ± 1 | |||||
Data presented as mean ± SD (n = 3). All the compounds and the standard dissolved in DMSO, diluted with culture medium containing 0.1% DMSO. The control cells were treated with culture medium containing 0.1% DMSO.
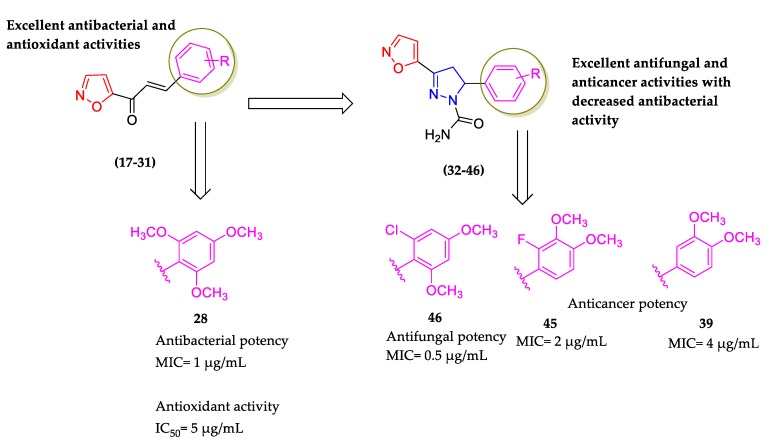
A summary of the antibacterial, antifungal, antioxidant, and cytotoxic activity of chalcones (17–31) and dihydropyrazoles (32–46) is depicted in Figure 3.
Figure 3.
Summarization of the antibacterial, antifungal, antioxidant, and cytotoxic activities of chalcones (17–31) and dihydropyrazoles (32–46).
3. Materials and Methods
3.1. Biological Activity Studies
3.1.1. Antibacterial and Antifungal Activities
The antibacterial and antifungal activity of the novel chalcones (17 to 31) and dihydropyrazoles (32 to 46) against the selected bacterial and fungal strains was assessed by following the procedure described in our previous paper [10].
3.1.2. Antioxidant Activity
The use of the DPPH assay provides an easy and rapid way to evaluate antioxidants by spectrophotometry, hence it can be useful to assess various products at a time. The purpose of this study was to evaluate the antioxidant activity of chalcones and pyrazolines using the DPPH free radical assay. The percentage of antioxidant activity (AA%) of all the compounds was assessed by the DPPH free radical assay. The measurement of the DPPH radical scavenging activity was performed according to methodology described by Brand-Williams et al. The samples were reacted with the stable DPPH radical in an ethanol solution. A 0.1 mM solution of DPPH was prepared by dissolving DPPH in methanol. Gallic acid was taken as a reference standard and different concentrations of test samples (5–100 µg/mL) and standard (1.0, 2.5, and 5.0 µg/mL) were prepared using methanol. One milliliter of 0.1 mM DPPH solution was added to 3 mL of all concentrations of test samples and standard separately. These mixtures were kept in dark for about 30 min and the absorbance was measured at 517 nm [49]. The capability to scavenge the DPPH radical was calculated using the formula:
| (1) |
when DPPH reacted with an antioxidant compound and was reduced, the change in color (from deep violet to light yellow) was read [Absorbance (Abs)] at 517 nm after 100 min of reaction using a UV-VIS spectrophotometer.
3.1.3. Anticancer Activity
The in vitro anticancer activity of chalcones (17 to 31) and dihydropyrazoles (32 to 46) was evaluated by the Mosmann’s MTT assay as described previously [34].
4. Conclusions
In a nutshell, we reported the antibacterial, antifungal, antioxidant, and anti-prostate cancer and structure–activity relationship studies of 30 isoxazole and substituted phenyl ring-containing compounds, including 15 chalcones and dihydropyrazoles. All the compounds were found to be nontoxic against the human normal cell line LO2. Biological screening data indicated that chalcones exhibited excellent antibacterial and antioxidant activities whereas the dihydropyrazole derivatives showed superior antifungal and anticancer activities. It was observed that the electronic property (electron withdrawing and electron releasing) of the substituents on the phenyl ring was instrumental for the difference in the potency of the compounds. For instance, chalcone 28 containing the 2,4,6-trimethoxy phenyl ring showed the potent antibacterial activity as well as antioxidant activity whereas dihydropyrazole 46 bearing the 2-chloro-4,6-dimethoxyphenyl was the potent antifungal compound and 45 and 39 having 2-fluoro-3,4-dimethoxyphenyl and 3,4-dimethoxyphenyl substituents showed excellent anticancer activity against the prostate cancer (DU-145) cell line. These findings have identified potential lead molecules against the proposed activities. Further studies are under progress to assess the influence of the bioisosteric replacement of the isoxazole scaffold with other privileged five-membered heterocyclic rings.
Acknowledgments
The authors like to acknowledge the managements of Vignan Pharmacy College, Vadlamudi, Andhra Pradesh, India and ASN Pharmacy College, Tenali, Andhra Pradesh, India for providing the required space and chemicals for this work. R.R.B would like to thank the Dean’s office of College of Pharmacy and Health Sciences, Ajman University, UAE for their support in preparation of this manuscript.
Author Contributions
Conceptualization, A.S., K.P., S.N. and V.K.; methodology, A.S., R.R.B., K.P., S.N., S.S.; software, A.S., R.R.B., K.P., S.N., S.S.; validation, A.S., R.R.B., K.P., S.N., V.K., and S.S.; formal analysis, A.S., R.R.B., K.P. and V.K.; investigation, A.S., K.P.; resources, A.S., K.P., S.N., and S.S.; data curation, A.S., K.P., S.N. and S.S.; writing—original draft preparation, A.S., R.R.B., K.P., S.N. and S.S.; writing—review and editing, A.S., R.R.B., K.P., S.N. and S.S.; visualization, A.S., K.P. and V.K.; supervision, V.K.; project administration, A.S., K.P., S.N., V.K. and S.S.; funding acquisition, A.S., R.R.B., K.P.,V.K. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Gibson M.Z., Nguyen M.A., Zingales S.K. Design, synthesis and evaluation of (2-(Pyridinyl)methylene)-1-tetralone chalcones for Anticancer and Antimicrobial Activity. Med. Chem. 2018;14:333–343. doi: 10.2174/1573406413666171020121244. [DOI] [PubMed] [Google Scholar]
- 2.Gao Z., Hurst W.J., Czechtizky W., Hall D., Moindrot N., Nagorny R., Pichat P., Stefany D., Hendrix J.A., George P.G. Identification and profiling of 3,5-dimethyl-isoxazole-4-carboxylic acid [2-methyl-4-((2S,3′S)-2-methyl-[1,3′]bipyrrolidinyl-1′-yl)phenyl] amide as histamine H(3) receptor antagonist for the treatment of depression. Bioorg. Med. Chem. Lett. 2013;23:6269–6273. doi: 10.1016/j.bmcl.2013.09.081. [DOI] [PubMed] [Google Scholar]
- 3.Afzal B.S., Lohitha S.V.K., Puttagunta S.B., Shaik A., Supraja K., Sai H.K. Synthesis and screening of novel lipophilic diarylpropeones as prospective antitubercular, antibacterial and antifungal agents. Biointerface Res. Appl. Chem. 2019;9:3912–3918. [Google Scholar]
- 4.RamirezPrada J., Robledo S.M., Velez I.D., Crespo M.D.P., Quiroga J., Abonia R., Montoya A., Svetaz L., Zacchino S., Insuasty B. Synthesis of novel quinoline-based 4,5-dihydro-1-H-pyrazoles as potential anticancer, antifungal, antibacterial, antiprotozoal agents. Med. Chem. 2017;131:237–254. doi: 10.1016/j.ejmech.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Sowmya D.V., Lakshmi Teja A., Padmaja A., Kamala Prasad V., Padmavathi V. Green approach for the synthesis of thiophenyl pyrazoles and isoxazoles by adopting 1,3-dipolar cycloaddition methodology and their antimicrobial activity. Eur. J. Med. Chem. 2018;143:891–898. doi: 10.1016/j.ejmech.2017.11.093. [DOI] [PubMed] [Google Scholar]
- 6.Chunlin Z., Wen Z., Chunquan S., Wannian Z., Chengguo X., Zhenyuan M. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017;117:7762–7810. doi: 10.1021/acs.chemrev.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puja J., Dharam P.P., Himangini B., Uma A. Chalcone and their Heterocyclic Analogue: A Review Article. J. Chem. Pharm. Res. 2018;10:160–173. [Google Scholar]
- 8.Ahmet Ö., Mehlika D.A., Belgin S., Hülya K.G., Handan A.K., Özlem A., Merve B. A New Series of Pyrrole-Based Chalcones: Synthesis and Evaluation of Antimicrobial Activity, Cytotoxicity, and Genotoxicity. Molecules. 2017;22:2112. doi: 10.3390/molecules22122112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pallepati K., Venkata R.K., Afzal B.S. Antitubercular evaluation of isoxazole appended 1-carboxamido-4,5-dihydro-1H-pyrazoles. J. Res. Pharm. 2019;23:156–163. [Google Scholar]
- 10.Afzal B.S., Yejella R.P., Shaik S. Design, Facile Synthesis, Characterization and Computational Evaluation of Novel Isobutylchalcones as Cytotoxic Agents: Part-A. FABAD J. Pharm. Sci. 2015;40:7–22. [Google Scholar]
- 11.Kishor P., Venkata K., Afzal B.S. Antitubercular Evaluation of Isoxazolyl Chalcones. RJPBCS. 2017;8:730–735. [Google Scholar]
- 12.Lokesh B.V.S., Prasad Y.R., Shaik A.B. Novel pyrimidine derivatives from 2,5-dichloro-3-acetylthienyl chalcones as antifungal, antitubercular and cytotoxic agents: Design, synthesis, biological activity and docking study. Asian J. Chem. 2019;19:310–321. doi: 10.2174/1871526519666181217120626. [DOI] [PubMed] [Google Scholar]
- 13.Afzal B.S., Yejella R.P., Shaik S. Synthesis, Antimicrobial, and Computational Evaluation of Novel Isobutylchalcones as Antimicrobial Agents. Int. J. Med. Chem. 2017;2017:1–14. doi: 10.1155/2017/6873924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavanya G., Mallikarjunareddy L., Padmavathi V., Padmaja A. Synthesis and antimicrobial activity of (1,4-phenylene)bis(arylsulfonylpyrazoles and isoxazoles) Eur. J. Med. Chem. 2014;73:187–194. doi: 10.1016/j.ejmech.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Abdelhamid A.O., EI Sayed I.E., Zaki Y.H., Hussein A.M., Mangoud M.M., Hosny M.A. Utility of 5-(furan-2-yl)-3-(p-tolyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide in the synthesis of heterocyclic compounds with antimicrobial activity. Biorg. Med. Chem. 2019;13:48. doi: 10.1186/s13065-019-0566-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan S.Y. Synthesis, antibacterial and antifungal activity of some new pyrazoline and pyrazole derivatives. Molecules. 2013;18:2683–2711. doi: 10.3390/molecules18032683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caliskan B., Sinoplu E., Ibis K., Akhan Guzelcan E., Cetin Ataly R., Banoglu E. Synthesis and cellular bioactivities of novel isoxazole derivatives incorporating an arylpiperazine moiety as anticancer agents. J. Enzyme Inhib. Med. Chem. 2018;33:1352–1361. doi: 10.1080/14756366.2018.1504041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filali I., Romdhane A., Znati M., Jannet H.B., Bouajila J. Synthesis of new harmine isoxazoles and evaluation of their potential anti-alzheimer, anti-inflammatory, and anticancer activities. Med. Chem. 2016;12:184–190. doi: 10.2174/157340641202160209104115. [DOI] [PubMed] [Google Scholar]
- 19.Jiabing W., Lili H., Chanchan C., Ge L., Jingwen X., Mengya S., Qian C., Wulan L., Wenfei H., Peihong Q., et al. Design, synthesis and biological evaluation of chalcone analogues with novel dual antioxidant mechanisms as potential anti-ischemic stroke agents. Acta Pharm. Sin. B. 2019;9:335–350. doi: 10.1016/j.apsb.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elfi S.V.H., Widiastuti A.E.S. A green synthesis of chalcones as an antioxidant and anticancer. IOP Conf. Ser. Mater. Sci. Eng. 2018;299:012077. [Google Scholar]
- 21.Yesseny A.V.M., Maurico E.O., Diego A.S.M., Marcela A.C., Alejandra P.V., Elizabeth S., Marcela R., Susana A.Z., Carolina M., Claudia T., et al. Antimicrobial, Anti-Inflammatory and Antioxidant Activities of Polyoxygenated Chalcones. J. Braz. Chem. Soc. 2019;30:286–304. [Google Scholar]
- 22.Chandrabose K., Narayana S.H., Narayana M., Sakthivel R., Uma V., Elangovan M., Devarajan K., Piyush T. Advances in chalcones with anticancer activities. Recent Pat Anticancer Drug Discov. 2015;10:97–115. doi: 10.2174/1574892809666140819153902. [DOI] [PubMed] [Google Scholar]
- 23.Yong J.P., Lu C.Z., Wu X. Potential anticancer agents. I. Synthesis of isoxazole moiety containing quinazoline derivatives and preliminarily in vitro anticancer activity. Anticancer Agents Med. Chem. 2015;15:131–136. doi: 10.2174/1871520614666140812105445. [DOI] [PubMed] [Google Scholar]
- 24.Jimi M.A., Raj K. 4,5-Dihydro-1H-pyrazole: An indispensable scaffold. J. Enzyme Inhib. Med. Chem. 2014;29:427–442. doi: 10.3109/14756366.2013.795956. [DOI] [PubMed] [Google Scholar]
- 25.Hai-Chao W., Xiao-Qiang Y., Tian-Long Y., Hong-Xia L., Zhong-Chang W., Hai-Liang Z. Design, Synthesis and Biological Evaluation of Benzohydrazide Derivatives Containing Dihydropyrazoles as Potential EGFR Kinase Inhibitors. Molecules. 2016;21:1012. doi: 10.3390/molecules21081012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ewelina S., Zenon P.C., Bogdan M., Andrzej P., Wojciech K. Chalcones and Dihydrochalcones Augment TRAIL-Mediated Apoptosis in Prostate Cancer Cells. Molecules. 2010;15:5336–5353. doi: 10.3390/molecules15085336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hongtian Z., Lei T., Chenghong Z., Baochu W., Pingrong Y., Dian H., Lifang Z., Yang Z. Synthesis of Chalcone Derivatives: Inducing Apoptosis of HepG2 Cells via Regulating Reactive Oxygen Species and Mitochondrial Pathway. Front. Pharmacol. 2019;10:1–13. doi: 10.3389/fphar.2019.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidhya C.D., Hareeshbabu E., Krishnakumar K. Synthesis of hybrid molecules of isoxazole derivatives in search of new anticancer drugs—A review. IJARIIT. 2019;5:1348–1355. [Google Scholar]
- 29.Vanessa G., Sidnei M., Alex F.C.F., Darlene C.F., Pio C., Ernani P. Antioxidant and Antimicrobial Properties of 2-(4,5-Dihydro-1H-pyrazol-1-yl)- pyrimidine and 1-Carboxamidino-1H-pyrazole Derivatives. J. Braz. Chem. Soc. 2010;21:1477–1483. [Google Scholar]
- 30.Havrylyuk D., Kovach N., Zimenkovsky B., Vasylenko O., Lesyk R. Synthesis and anticancer activity of isatin-based pyrazolines and thiazolidines conjugates. Arch. Pharm. 2011;344:514–522. doi: 10.1002/ardp.201100055. [DOI] [PubMed] [Google Scholar]
- 31.Insuasty B., Montoya A., Becerra D., Quiroga J., Abonia R., Robledo S., Velez I.D., Upegui Y., Nogueras M., Cobo J. Synthesis of novel analogs of 2-pyrazoline obtained from [(7-chloroquinolin-4-yl)amino]chalcones and hydrazine as potential antitumor and antimalarial agents. Eur. J. Med. Chem. 2013;67:252–262. doi: 10.1016/j.ejmech.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez J., Chicharro J., Bueno J.M., Lorenzo M. Isoxazole mediated synthesis of 4-(1H)pyridones: Improved preparation of antimalarial candidate GSK932121. Chem. Commun. (camb) 2016;52:10190–10192. doi: 10.1039/C6CC05277K. [DOI] [PubMed] [Google Scholar]
- 33.Bueno J.M., Herreros E., Angulo-Barturen I., Ferre S., Fiandor J.M., Gamo F.J., Gargallo-Viola D., Derimanov G. Exploration of 4(1H)-pyridones as a novel family of potent antimalarial inhibitors of the plasmodial cytochrome bc1. Fut. Med. Chem. 2012;4:2311–2323. doi: 10.4155/fmc.12.177. [DOI] [PubMed] [Google Scholar]
- 34.Patel P., Koregaokar S., Shah M., Parekh H. Synthesis of some novel pyrazoline and cyanopyridine derivatives as antimicrobial agents. Farmaco. 1996;51:59–63. [PubMed] [Google Scholar]
- 35.Guan L.P., Zhao D.H., Chang Y., Wen Z.S., Tang L.M., Huang F.F. Synthesis of 2,4-dihydroxychalcone derivatives as potential antidepressant effect. Drug Res. (Stuttg) 2013;63:46–51. doi: 10.1055/s-0032-1333229. [DOI] [PubMed] [Google Scholar]
- 36.Yu L.F., Tuckmantel W., Eaton J.B., Caldarone B., Fedolak A., hanania T., Brunner D., Lukas R.J., Kozikowski A.P. Identification of novel α4β2-nicotinic acetylcholine receptor (nAChR) agonists based on an isoxazole ether scaffold that demonstrate antidepressant-like activity. J. Med. Chem. 2012;55:55812–55823. doi: 10.1021/jm201301h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J., Yu L.F., Eaton J.B., Caldarone B., Cavino K., Ruiz C., Terry M., Fedolak A., Wang D., Ghavami A., et al. Discovery of isoxazole analogues of sazetidine-A as selective α4β2-nicotinic acetylcholine receptor partial agonists for the treatment of depression. J. Med. Chem. 2011;54:7280–7288. doi: 10.1021/jm200855b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajendra Prasad Y., Lakshmana Rao A., Prasoona L., Murali K., Ravi Kumar P. Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(2″-hydroxy naphthalen-1″-yl)-1,5-diphenyl-2-pyrazolines. Bioorg. Med. Chem. Lett. 2005;15:5030–5034. doi: 10.1016/j.bmcl.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 39.Palaska E., Aytemir M., Uzbay I.T., Erol D. Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur. J. Med. Chem. 2001;36:539–543. doi: 10.1016/S0223-5234(01)01243-0. [DOI] [PubMed] [Google Scholar]
- 40.Lin Y.M., Zhou Y., Flavin M.T., Zhou L.M., Nie W., Chen F.C. Chalcones and flavonoids as anti-tuberculosis agents. Bioorg. Med. Chem. 2002;10:2795–2802. doi: 10.1016/S0968-0896(02)00094-9. [DOI] [PubMed] [Google Scholar]
- 41.Azzali E., Machado D., Kaushik A., Vacondio F., Flisi S., Cabassi C.S., Lamichhane G., Viveiros M., Costantino G., Pieroni M. Substituted N-Phenyl-5-(2-(phenylamino)thiazol-4-yl)isoxazole-3-carboxamides are valuable antitubercular candidates that evade innate efflux machinery. J. Med. Chem. 2017;60:7108–7122. doi: 10.1021/acs.jmedchem.7b00793. [DOI] [PubMed] [Google Scholar]
- 42.Balaji N.V., HariBabu B., Rao V.U., Subbaraju G.V., Nagasree K.P., Kumar M.M.K. Synthesis, screening and docking analysis of hispolon pyrazoles and isoxazoles as potential antitubercular agents. Curr. Top. Med. Chem. 2019;19:662–682. doi: 10.2174/1568026619666190305124954. [DOI] [PubMed] [Google Scholar]
- 43.Lokesh B.V.S., Prasad Y.R., Shaik A.B. Synthesis, Biological evaluation and molecular docking studies of new pyrazolines as an antitubercular and cytotoxic agents. Infect. Disord. Drug Targets. 2019;19:310–321. doi: 10.2174/1871526519666181217120626. [DOI] [PubMed] [Google Scholar]
- 44.Dixit S.R., Joshi S.D., Kulkarni V.H., Jalalpure S.S., Kumbar V.M., Mudaraddi T.Y., Nadagouda M.N., Aminabhavi T.M. Pyrrolyl pyrazoline carbaldehydes as Enoyl-ACP reductase inhibitors. Design, synthesis and antitubercular activity. Open. Med. Chem. J. 2017;11:92–108. doi: 10.2174/1874104501711010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahapatra D.K., Bharti S.K., Asati V. Chalcone Derivatives: Anti-inflammatory Potential and Molecular Targets Perspectives. Curr. Top. Med. Chem. 2017;17:3146–3169. doi: 10.2174/1568026617666170914160446. [DOI] [PubMed] [Google Scholar]
- 46.Ozdemir A., Altintop M.D., Turan-Zitouni G., Çiftçi G.A., Ertorun I., Alataş O., Kaplancikli Z.A. Synthesis and evaluation of new indole-based chalcones as potential antiinflammatory agents. Eur. J. Med. Chem. 2015;89:304–309. doi: 10.1016/j.ejmech.2014.10.056. [DOI] [PubMed] [Google Scholar]
- 47.Gawad N.M., Georgey H.H., Ibrahim N.A., Amin N.H., Abdelsalam R.M. Synthesis of novel pyrazole and dihydropyrazoles derivatives as potential anti-inflammatory and analgesic agents. Chem. Commun. (Camb) 2016;52:14490–14493. doi: 10.1007/s12272-012-0507-y. [DOI] [PubMed] [Google Scholar]
- 48.Kharbanda C., Alam M.S., Hamid H., Javed K., Bano S., Dhulap A., Ali Y., Nazreen S., Haider S. Synthesis and evaluation of pyrazolines bearing benzothiazole as anti-inflammatory agents. Bioorg. Med. Chem. 2014;22:5804–5812. doi: 10.1016/j.bmc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 49.Eugenio J.G., Tatiane L.C.O., Severino M.A., Alessandra R., Alessandro D.L., Rosa H.M.G. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth, Braz. Dent. J. 2012;23:22–27. doi: 10.1590/s0103-64402012000100004. [DOI] [PubMed] [Google Scholar]